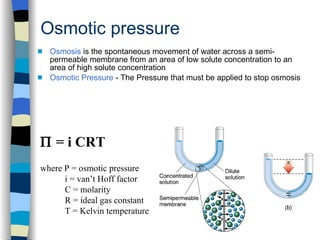
This document defines key terms related to solutions and summarizes factors that affect solubility. It defines a solution as a homogeneous mixture where a solute is dissolved in a solvent. Temperature, pressure, and the nature of the solute and solvent affect solubility. There are various units to express concentration, including molarity, molality, and percent composition. Colligative properties like boiling point elevation and freezing point depression depend on the number of solute particles rather than their identity.