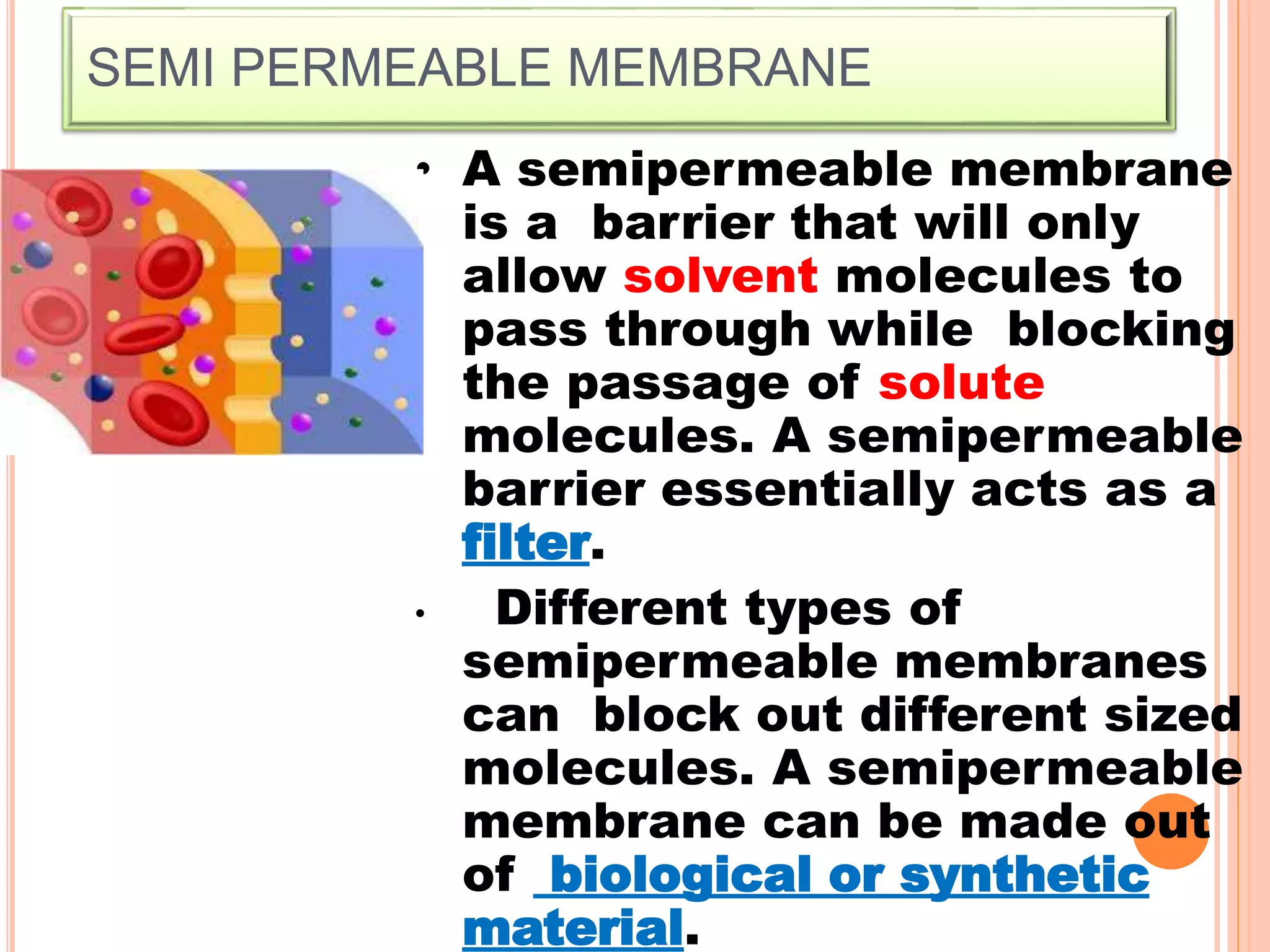
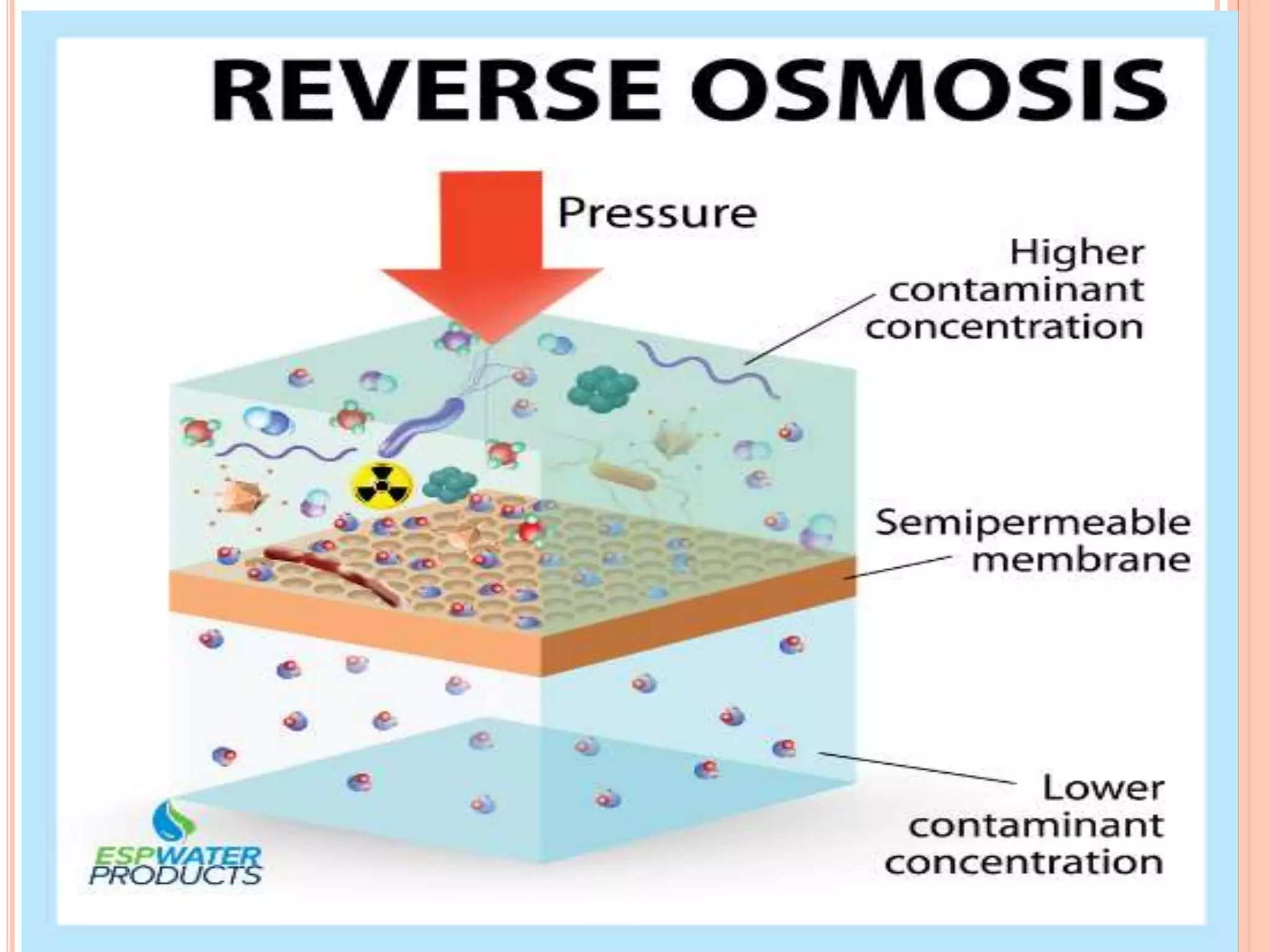
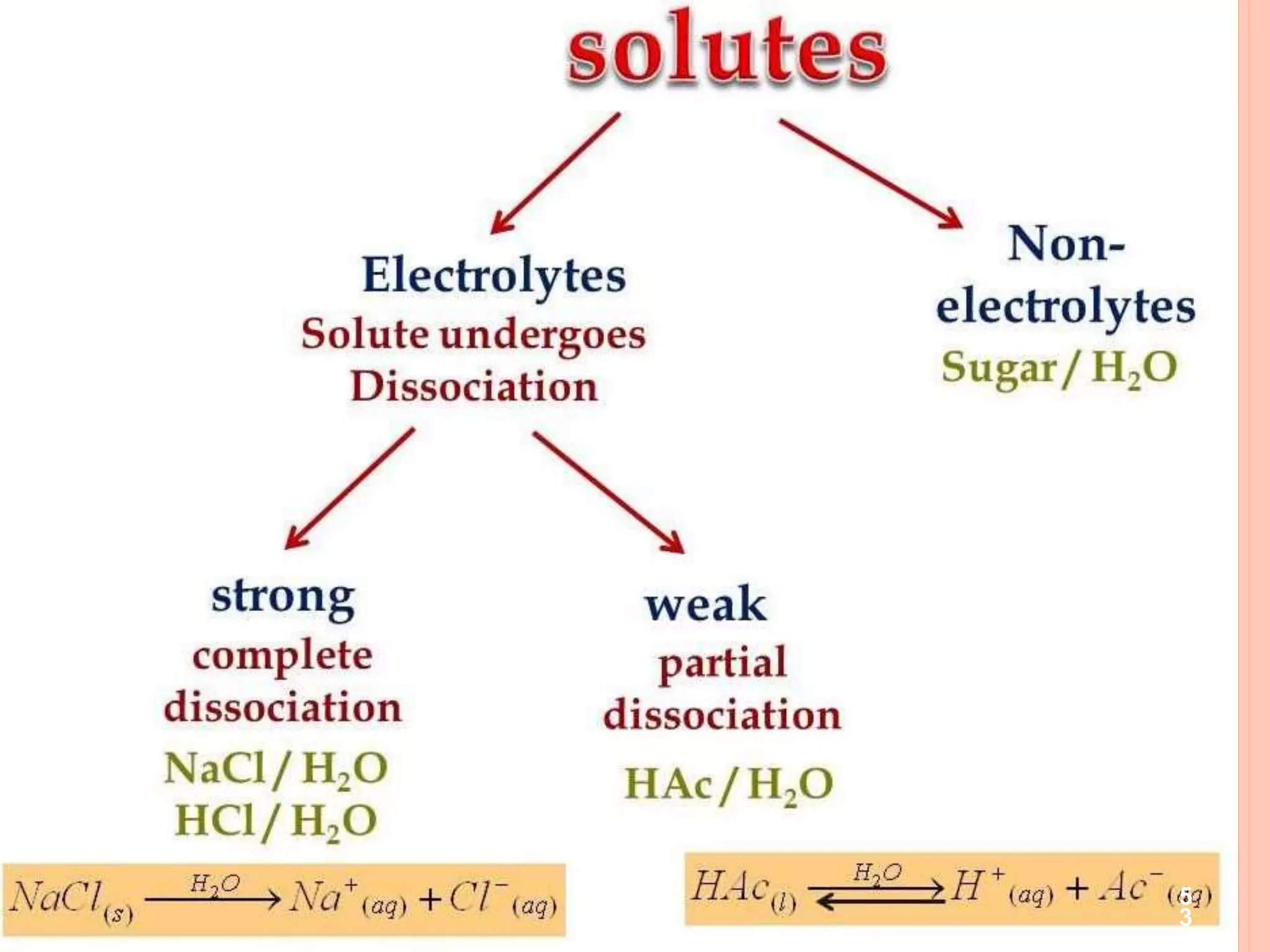
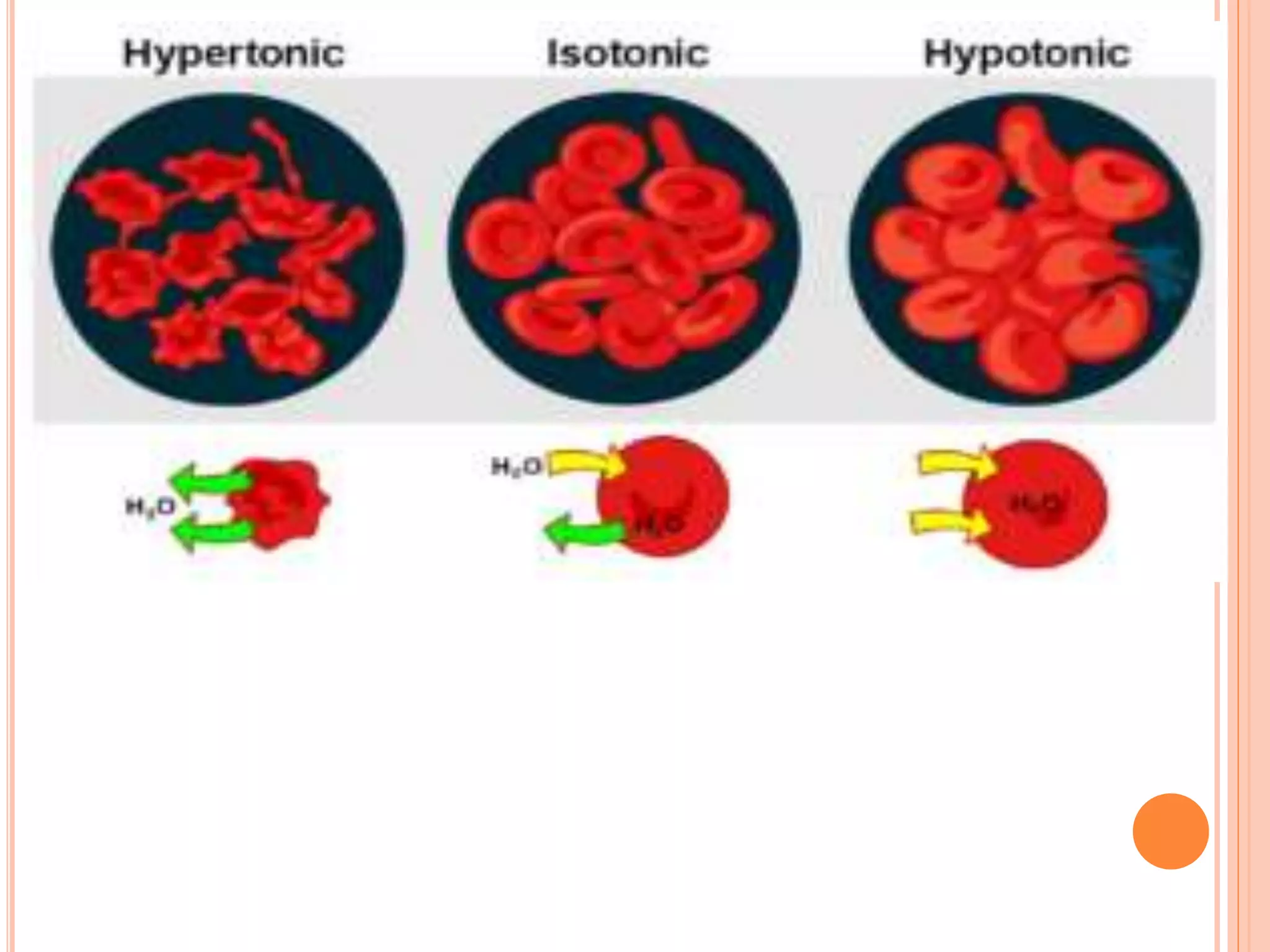
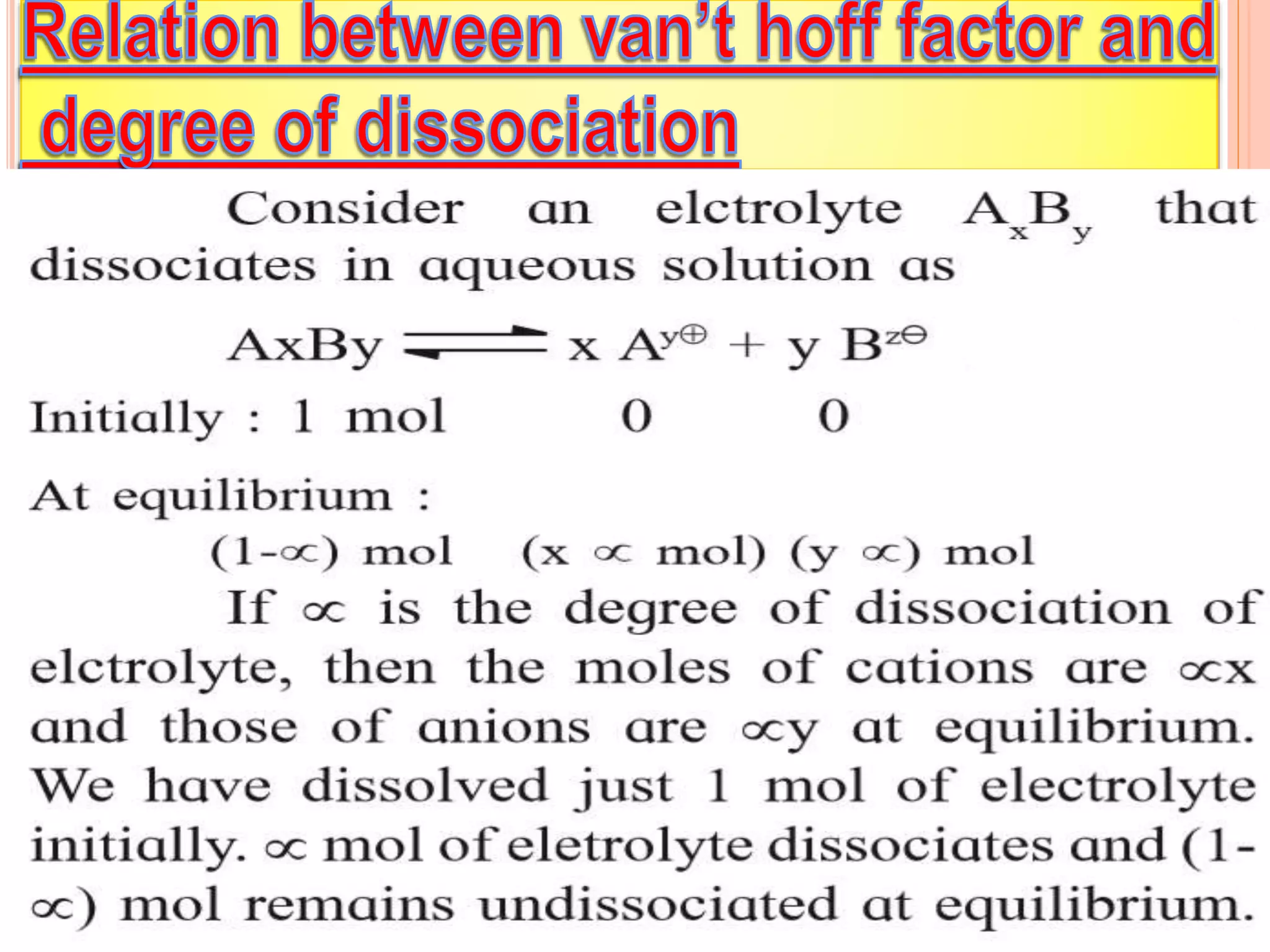
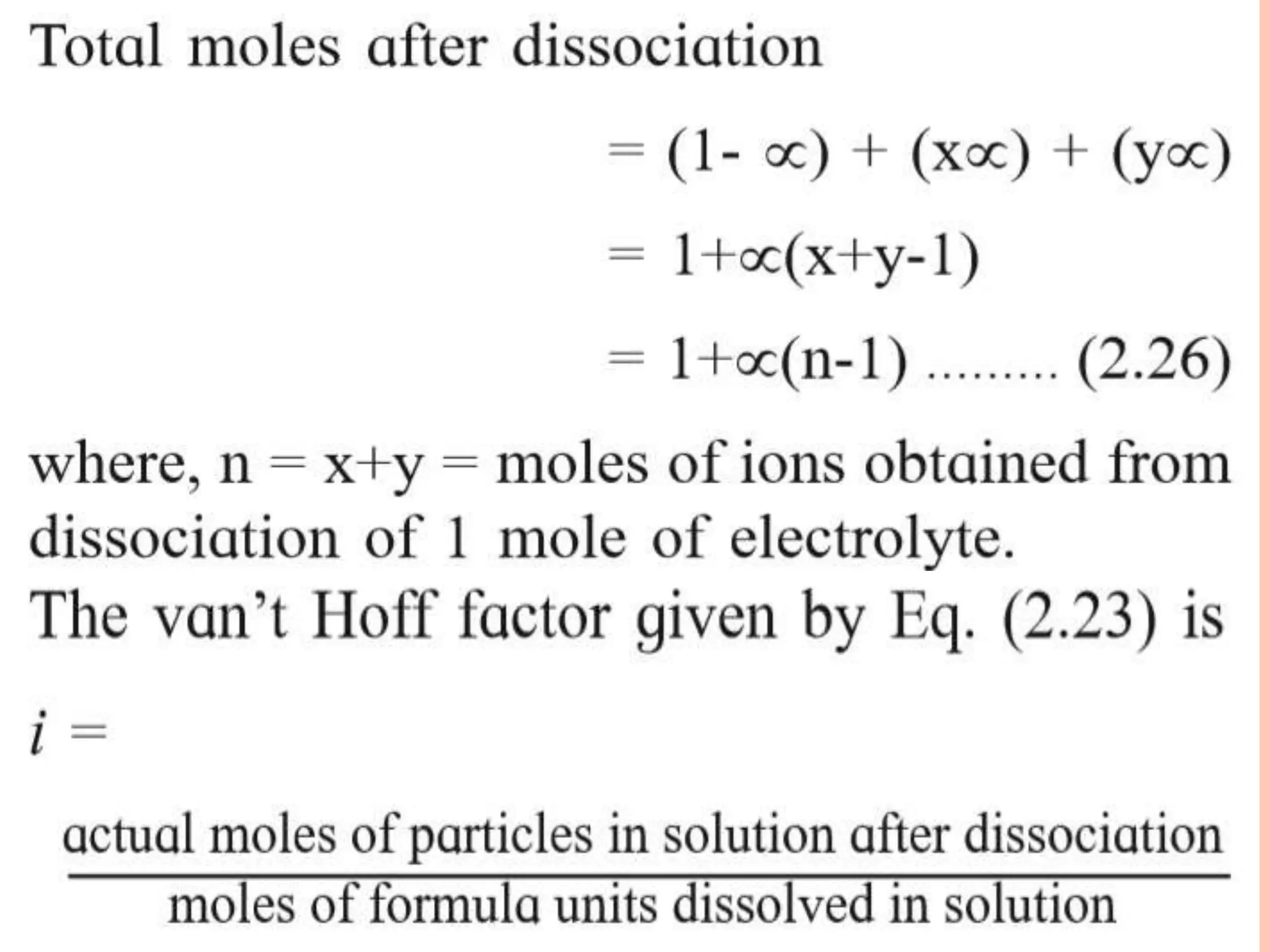
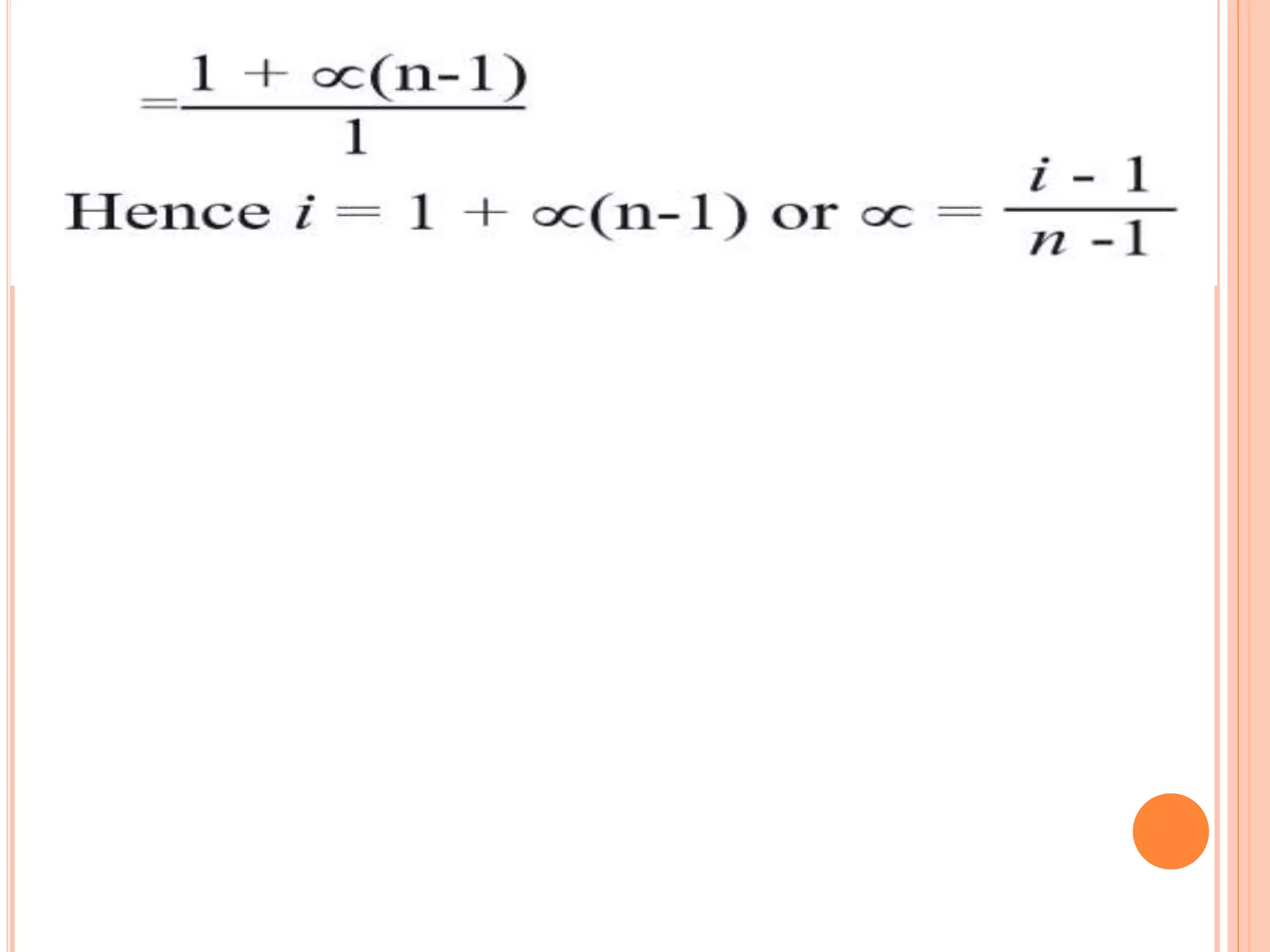The document discusses various concepts related to solutions including:
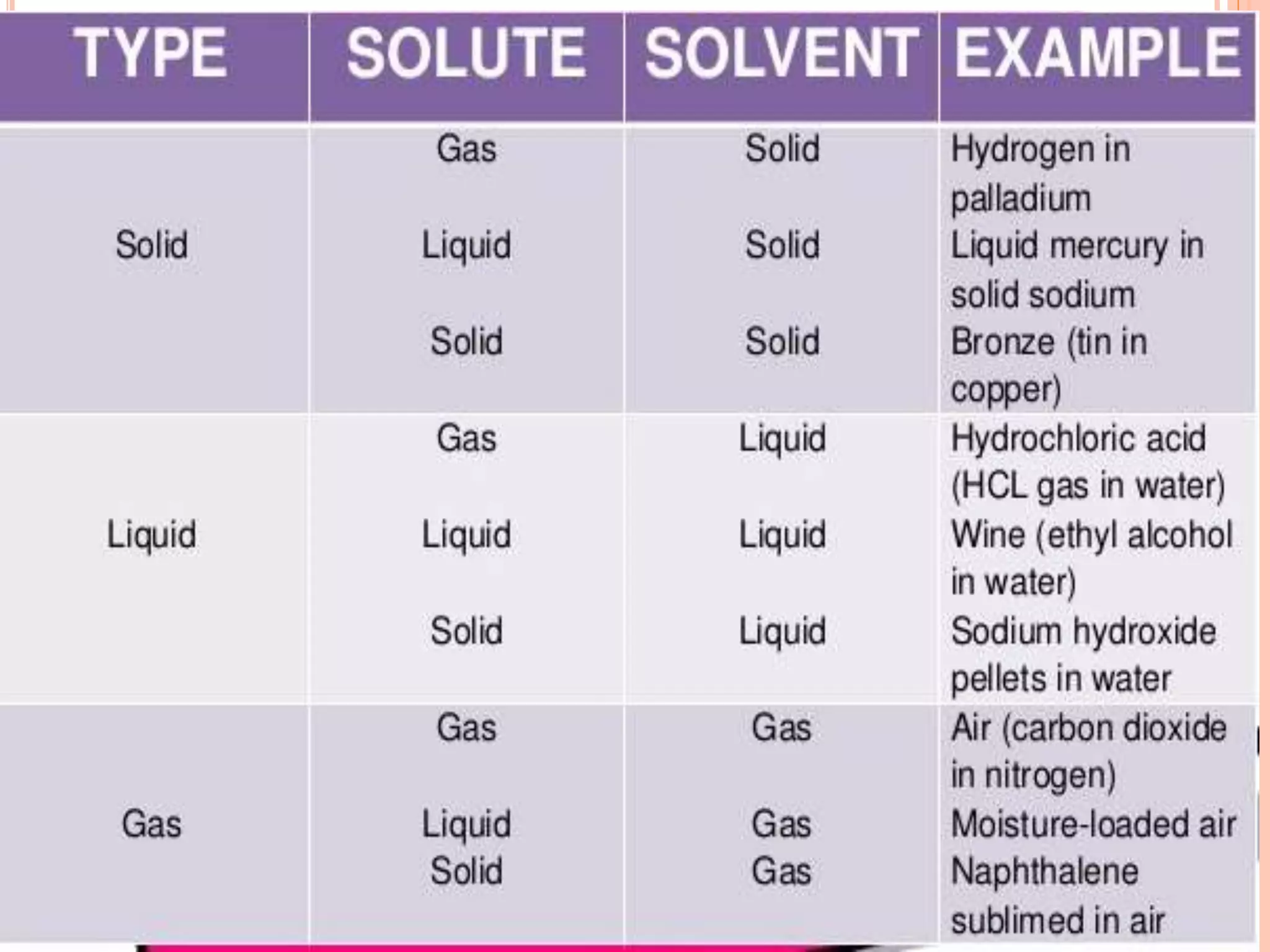
- Components of a solution including solvent, solute, and binary solutions.
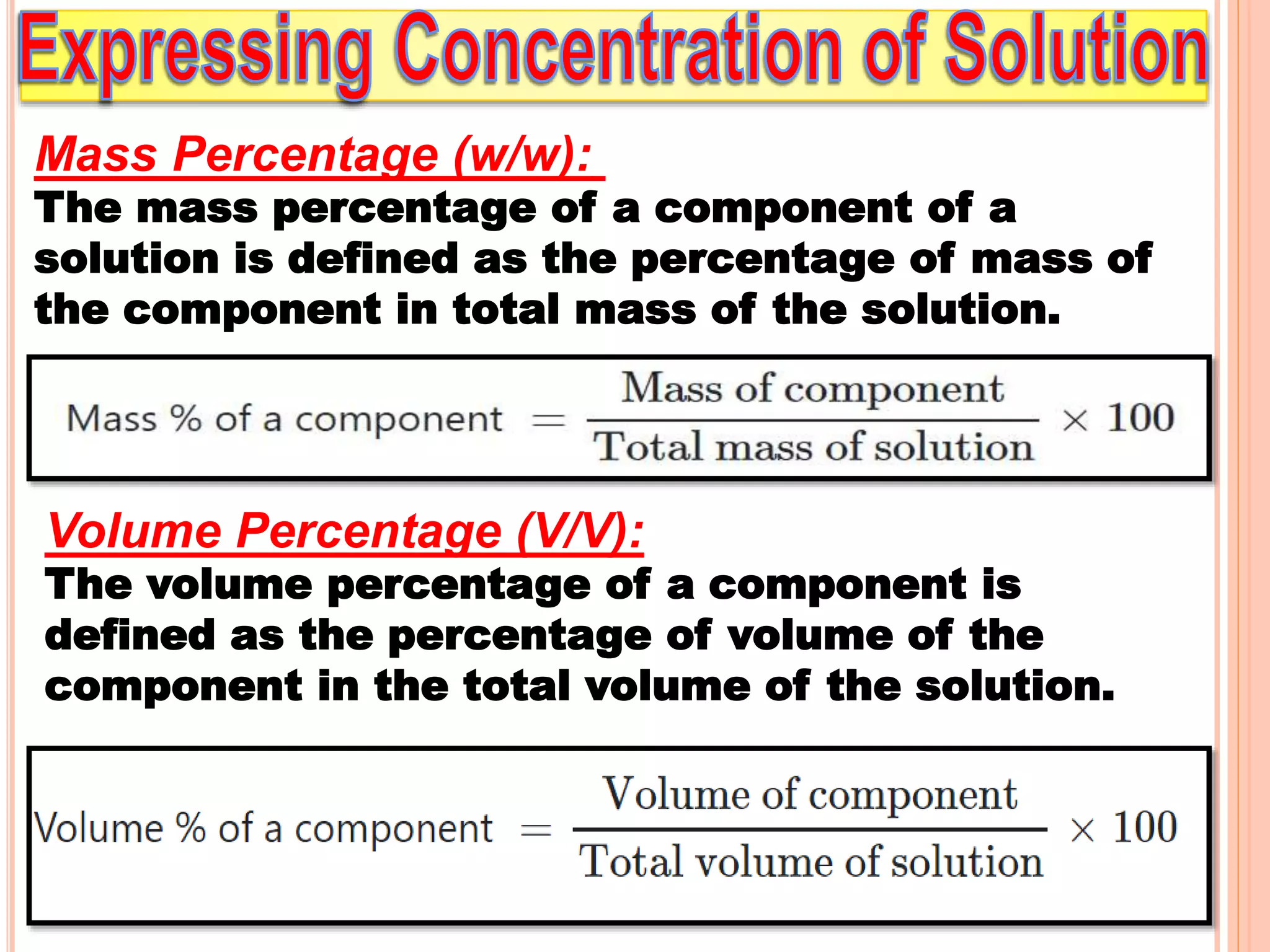
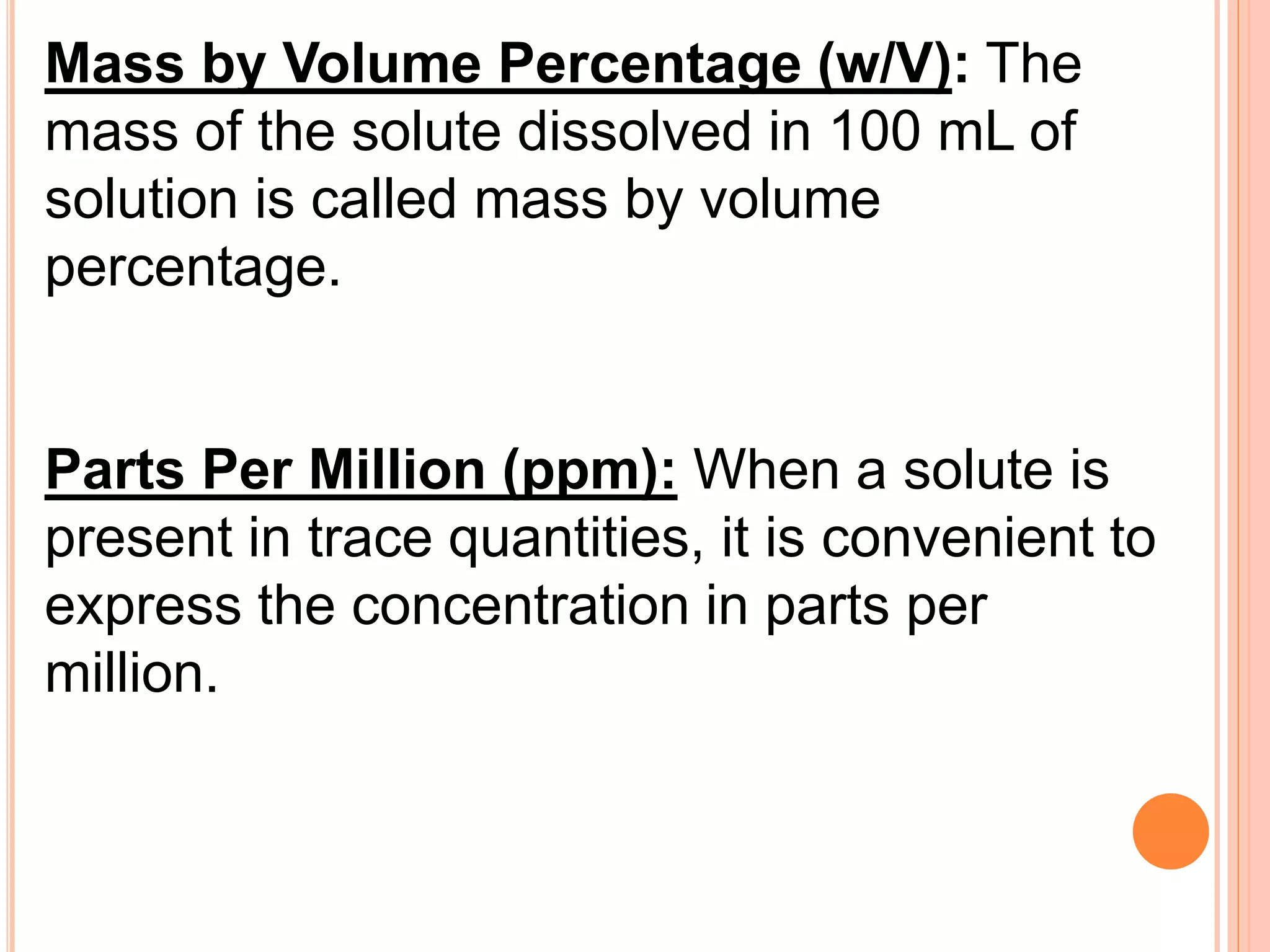
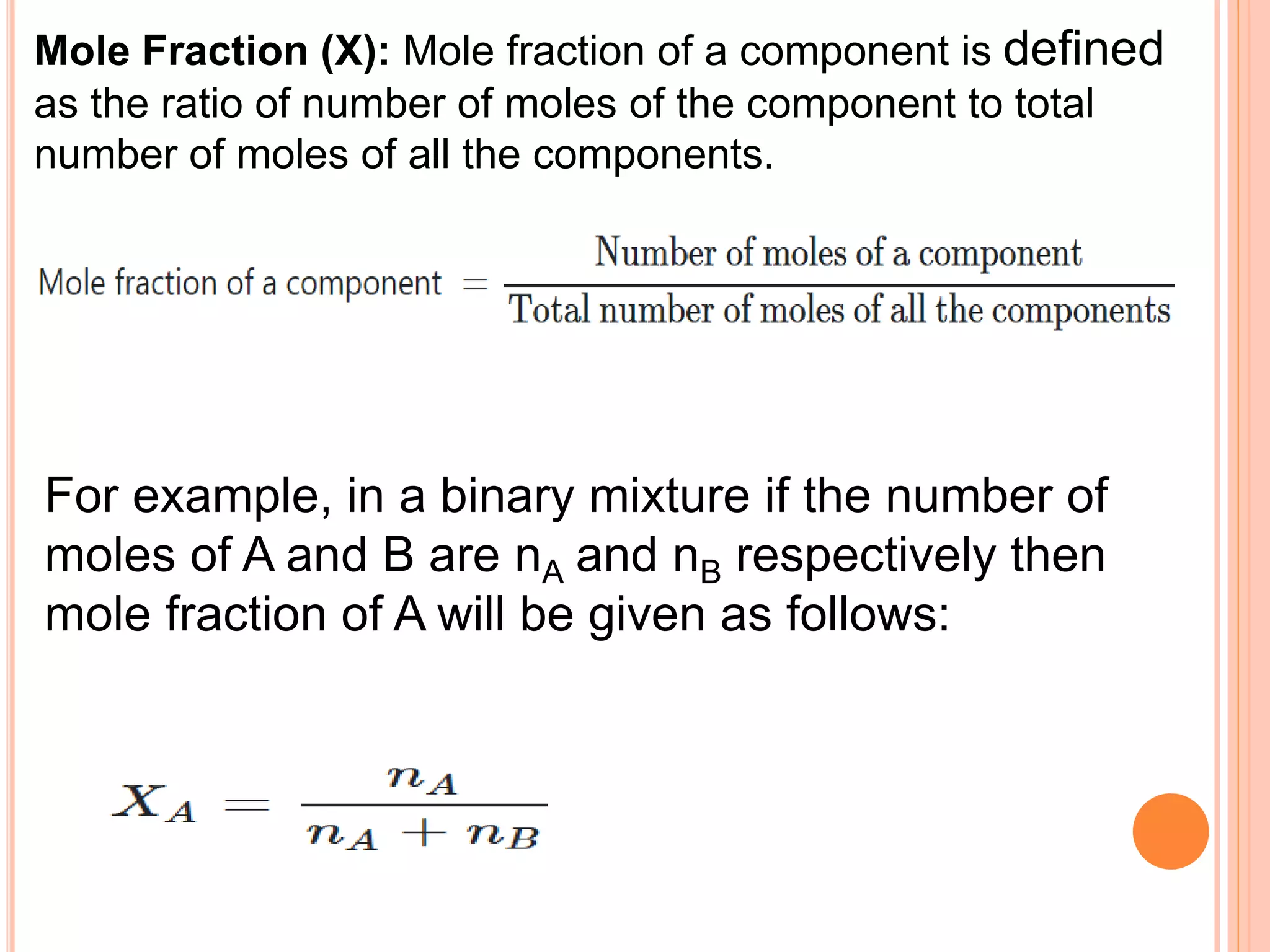
- Different units used to express concentration such as mass percentage, volume percentage, parts per million, and mole fraction.
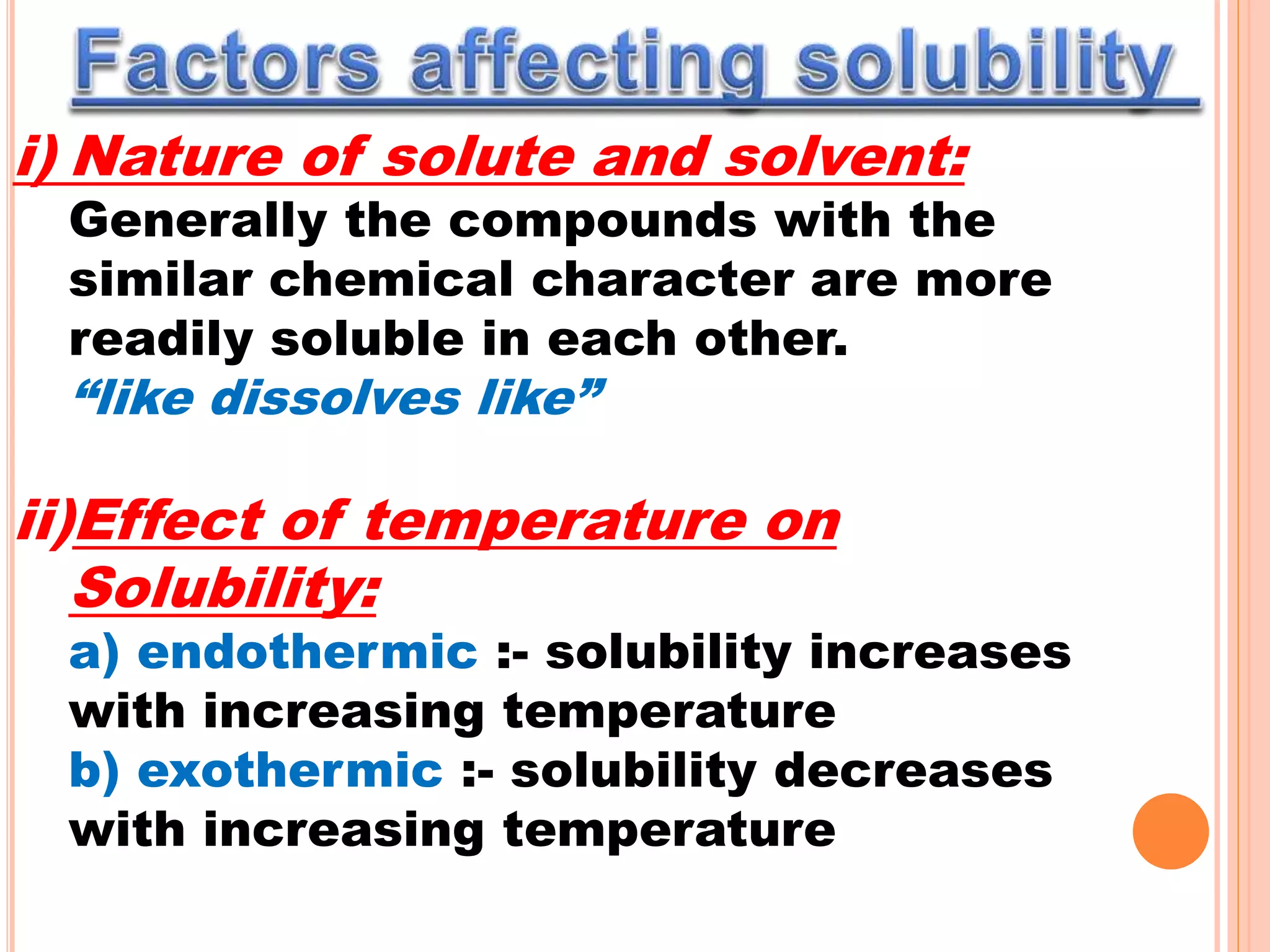
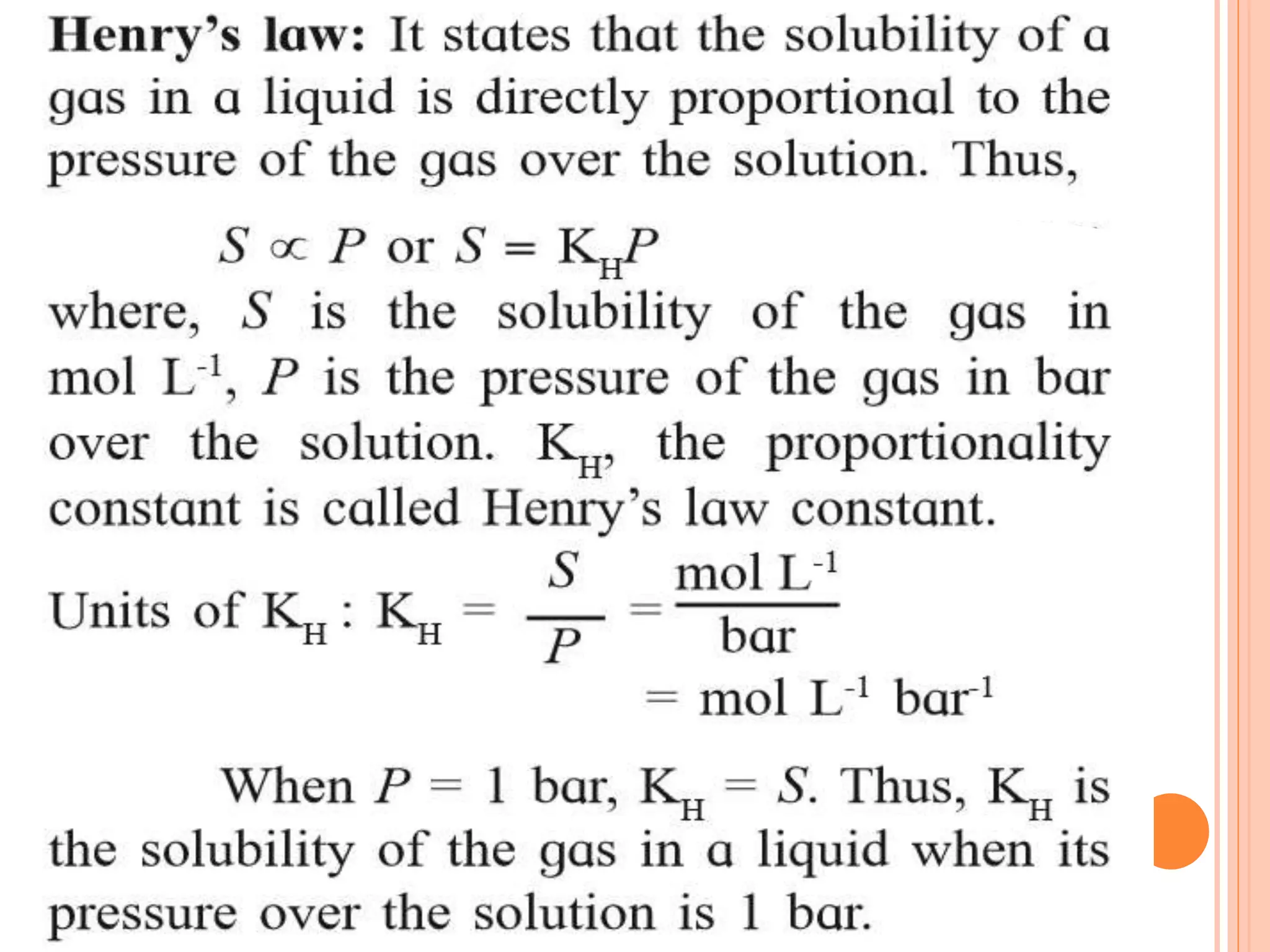
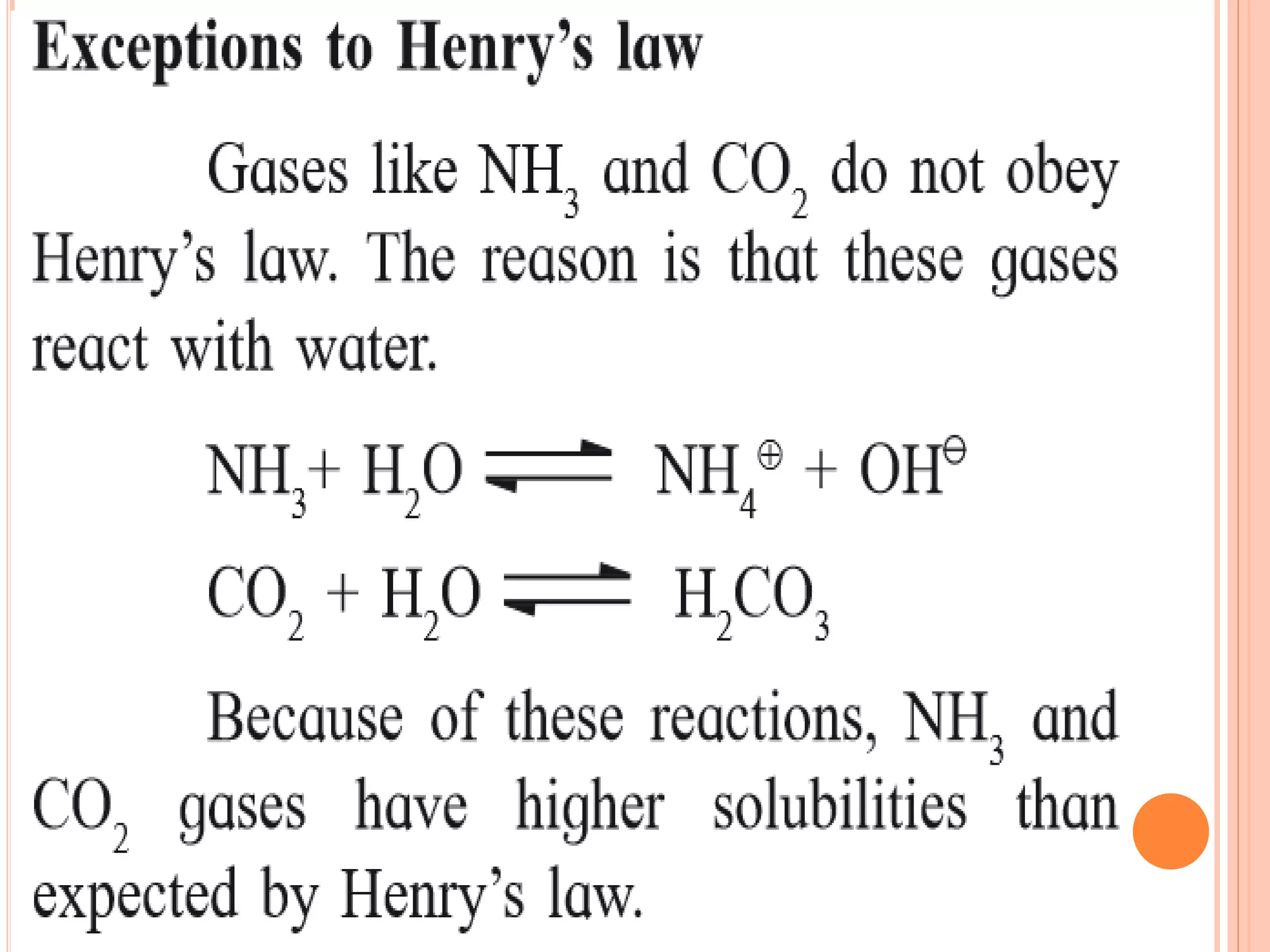
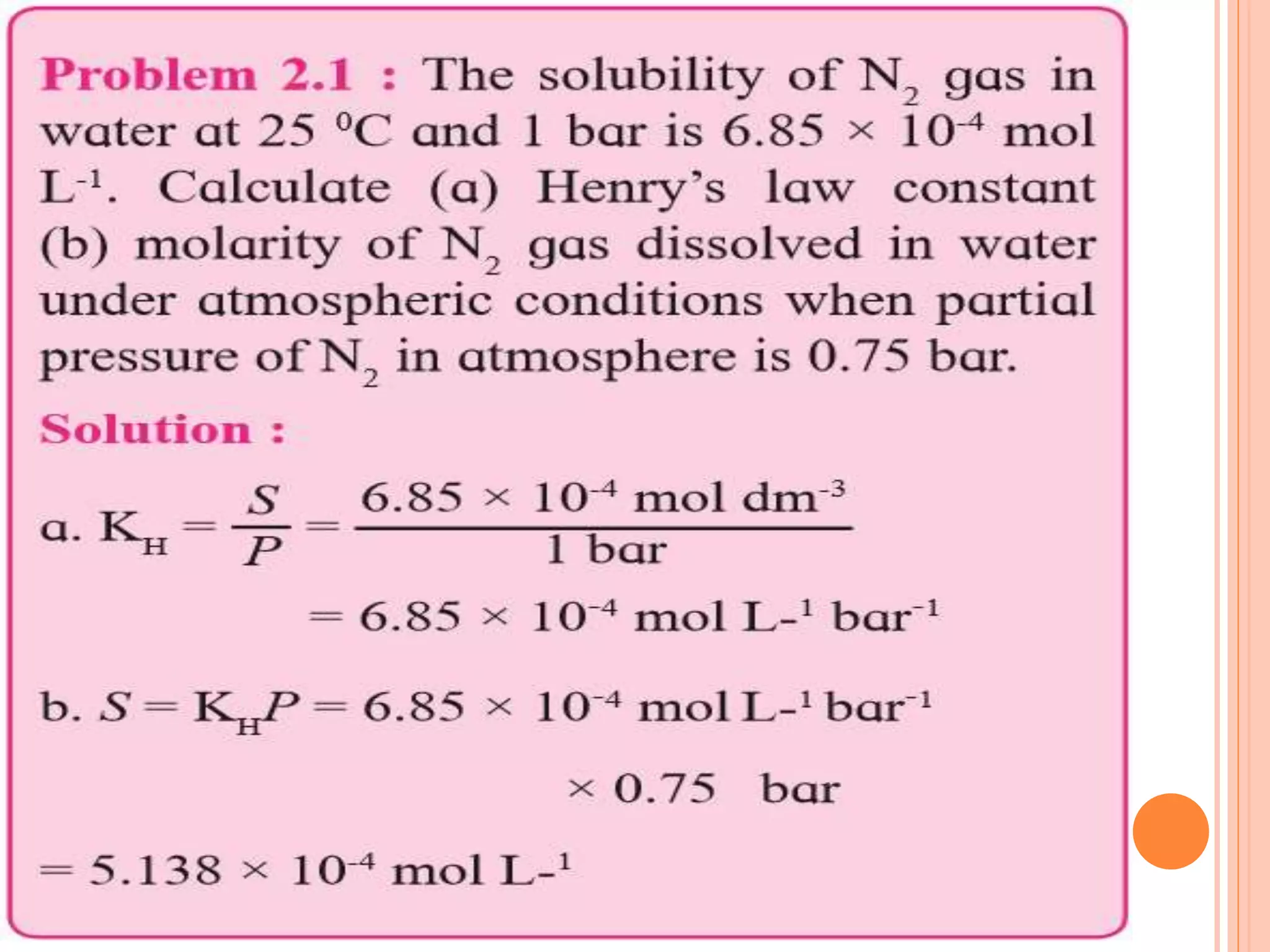
- Factors that affect solubility such as nature of solute and solvent, temperature, and pressure.
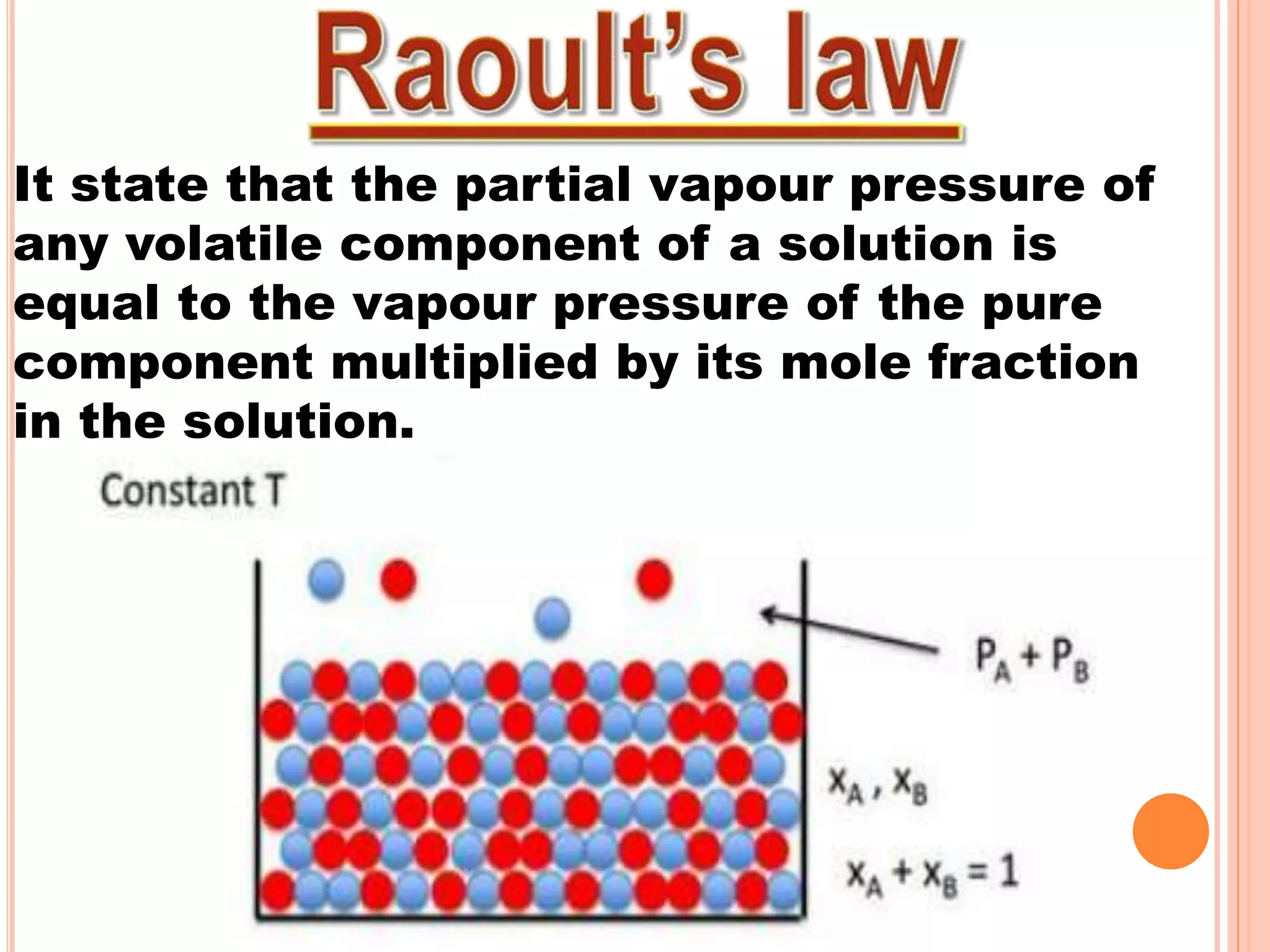
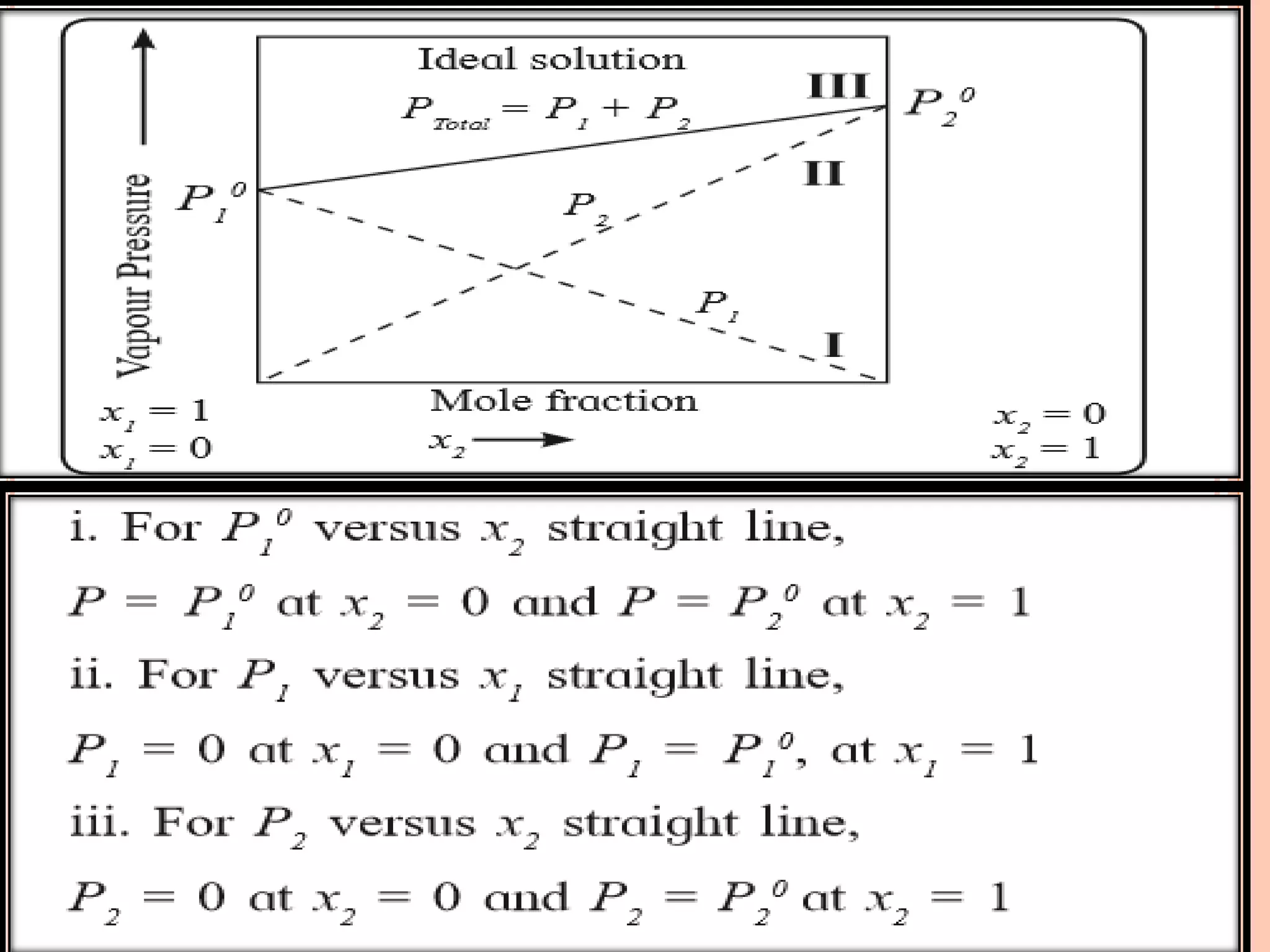
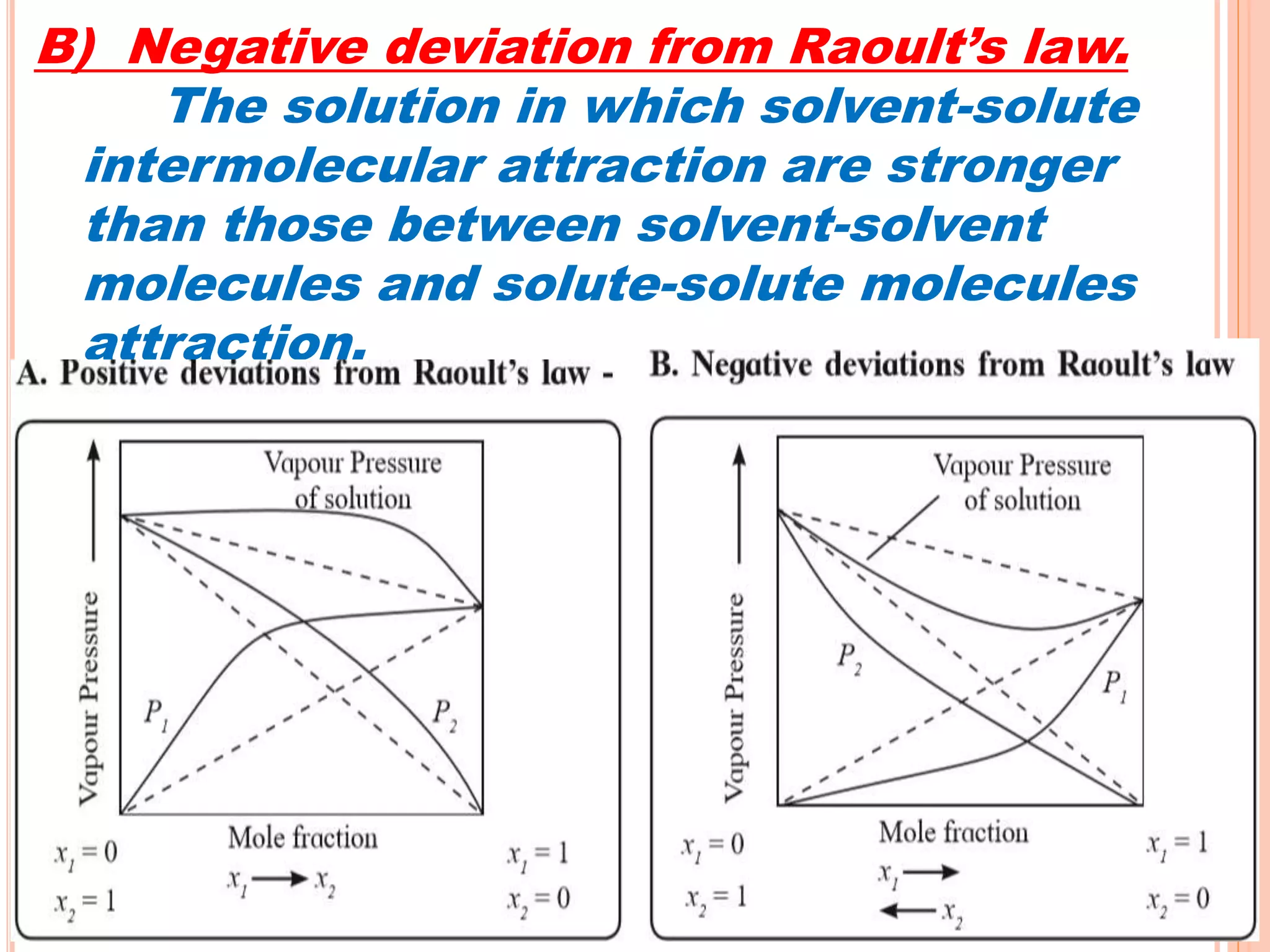
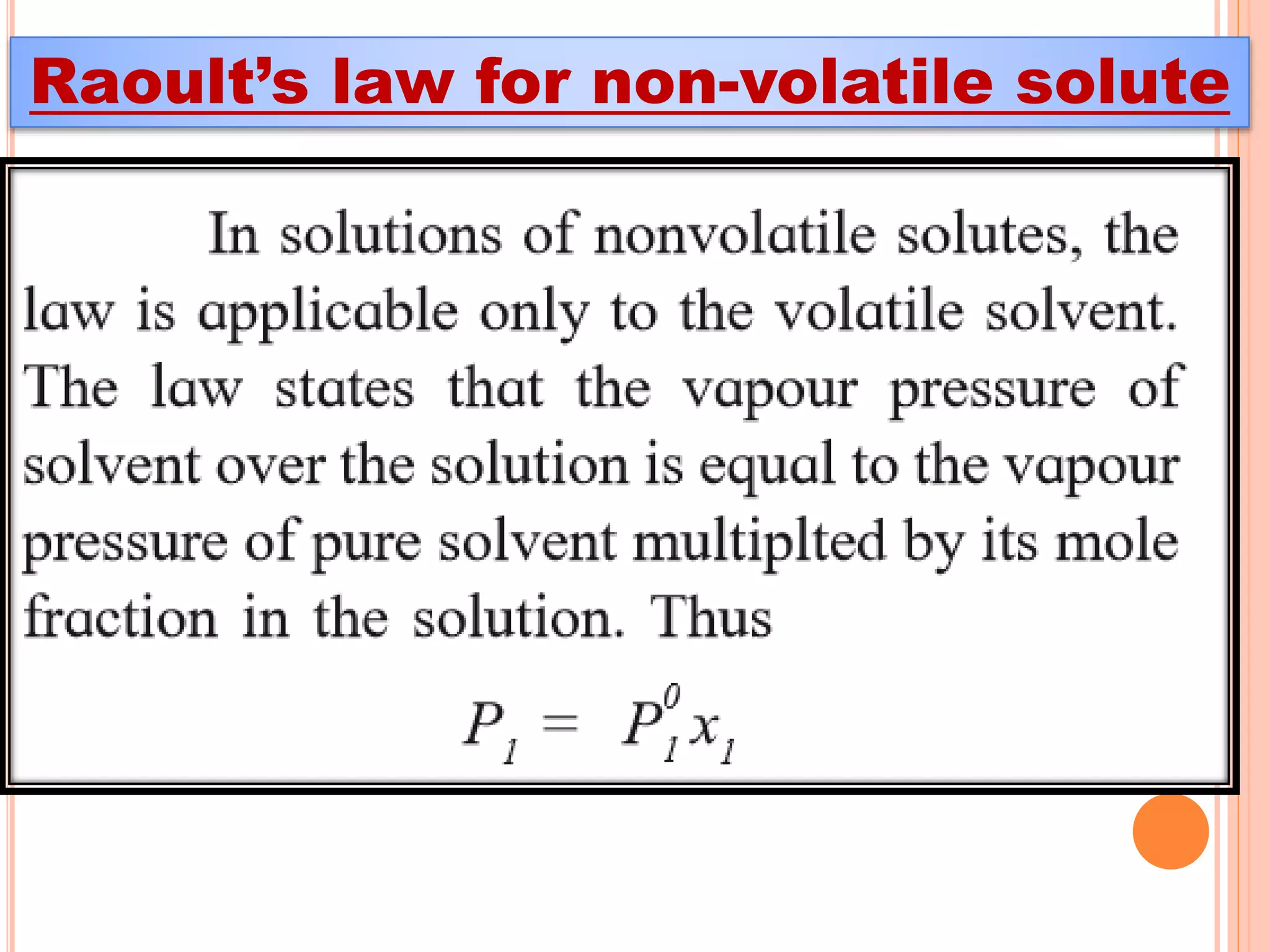
- Raoult's law and how it relates to ideal and non-ideal solutions.
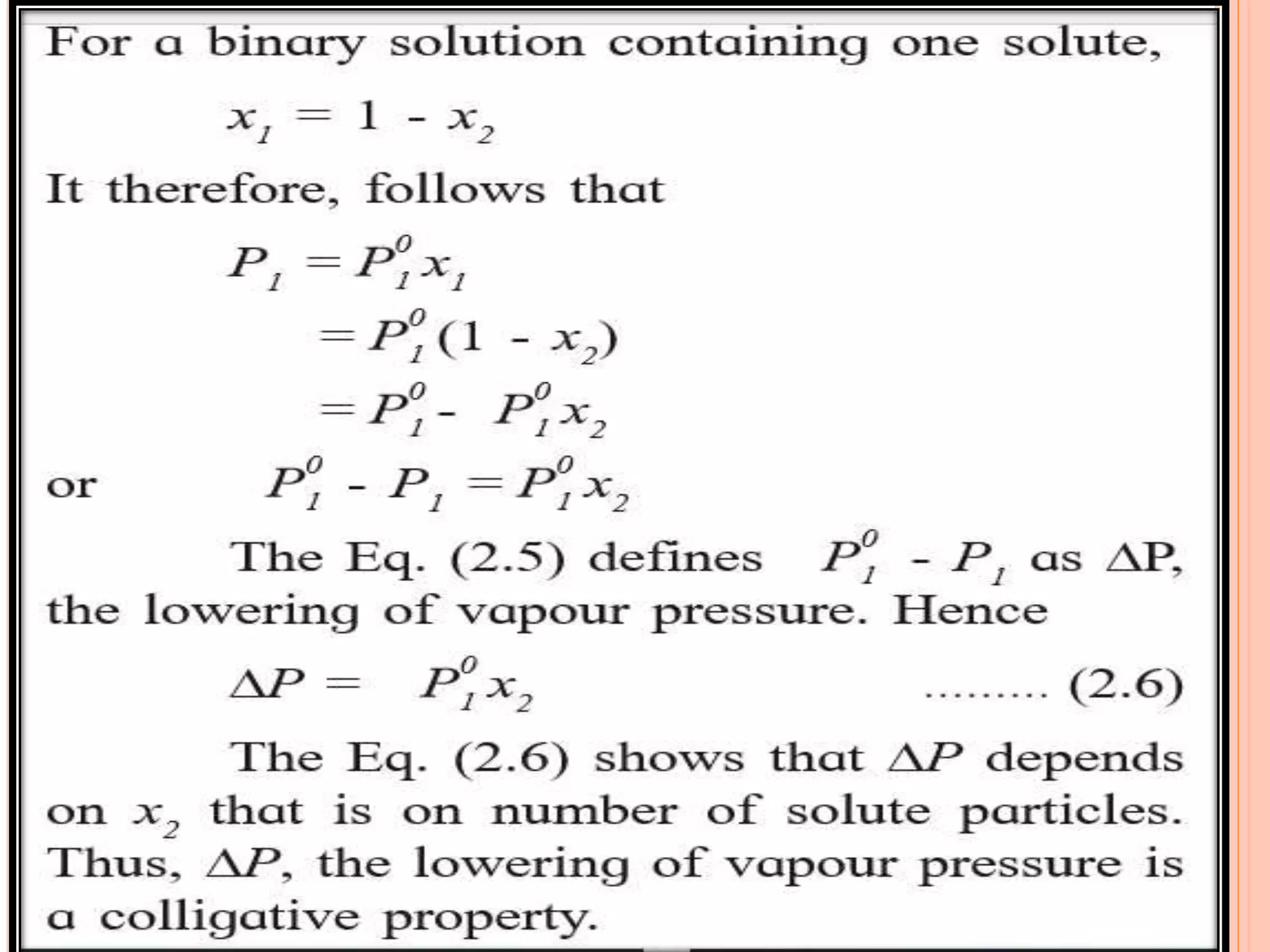
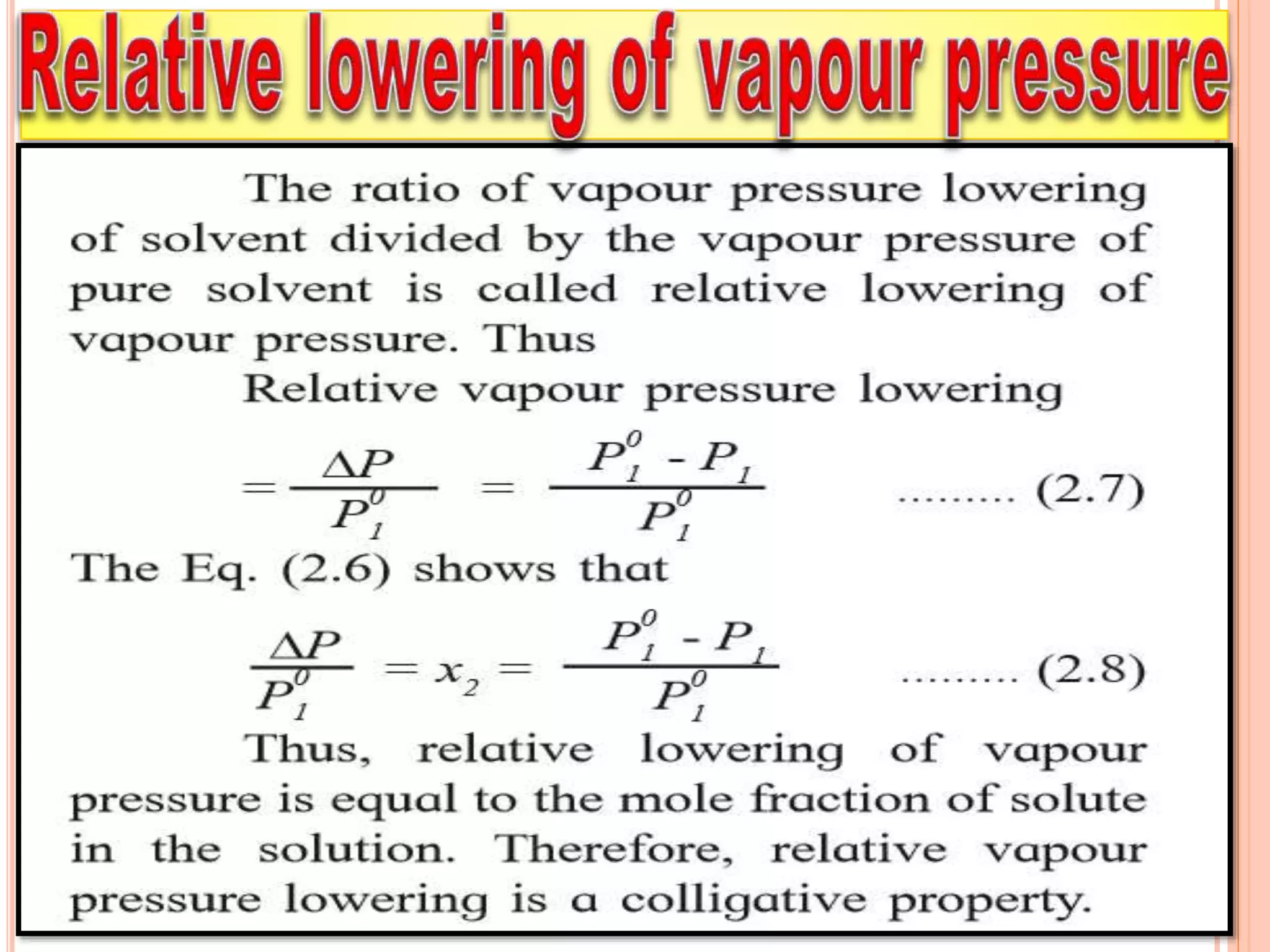
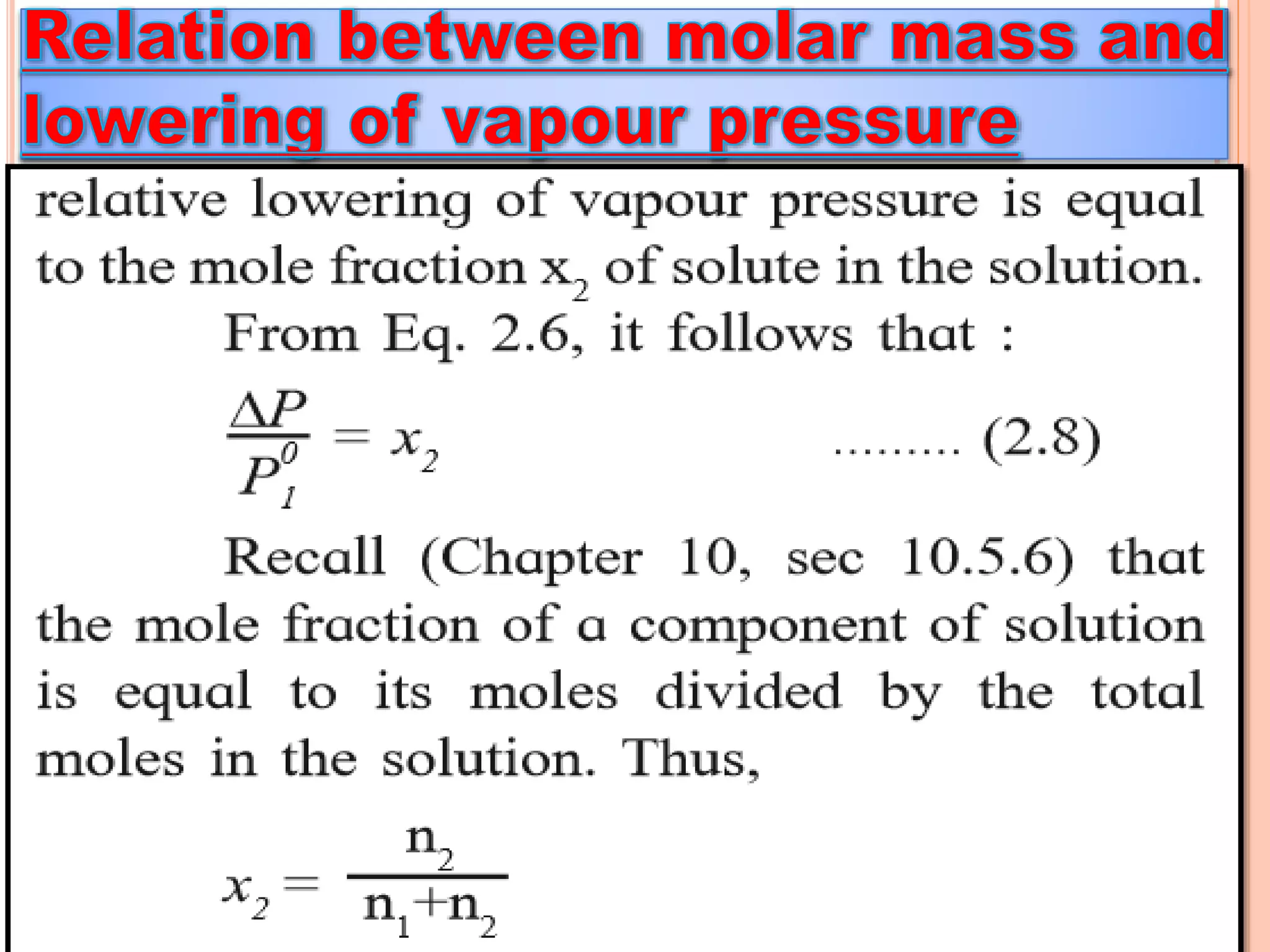
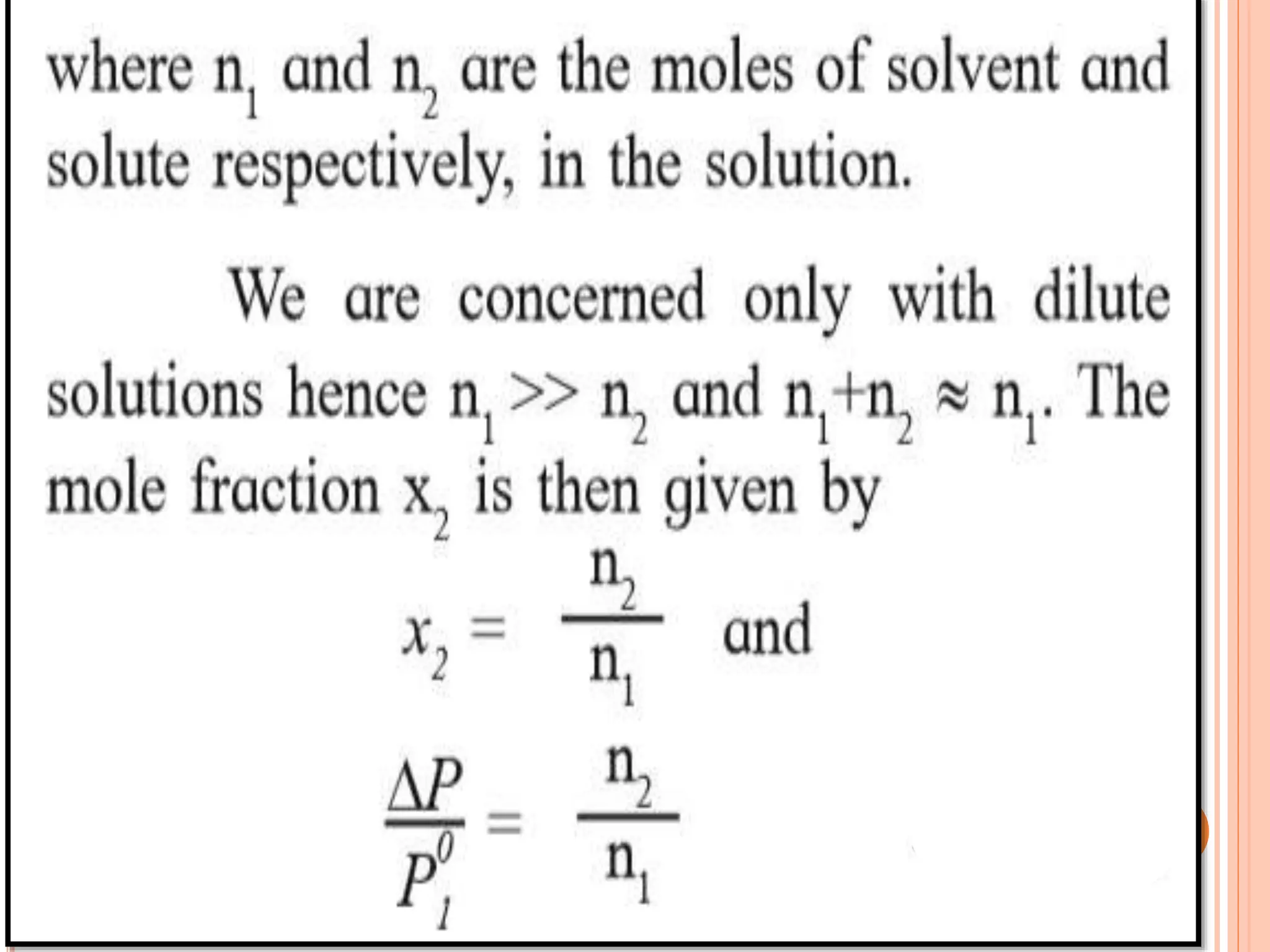
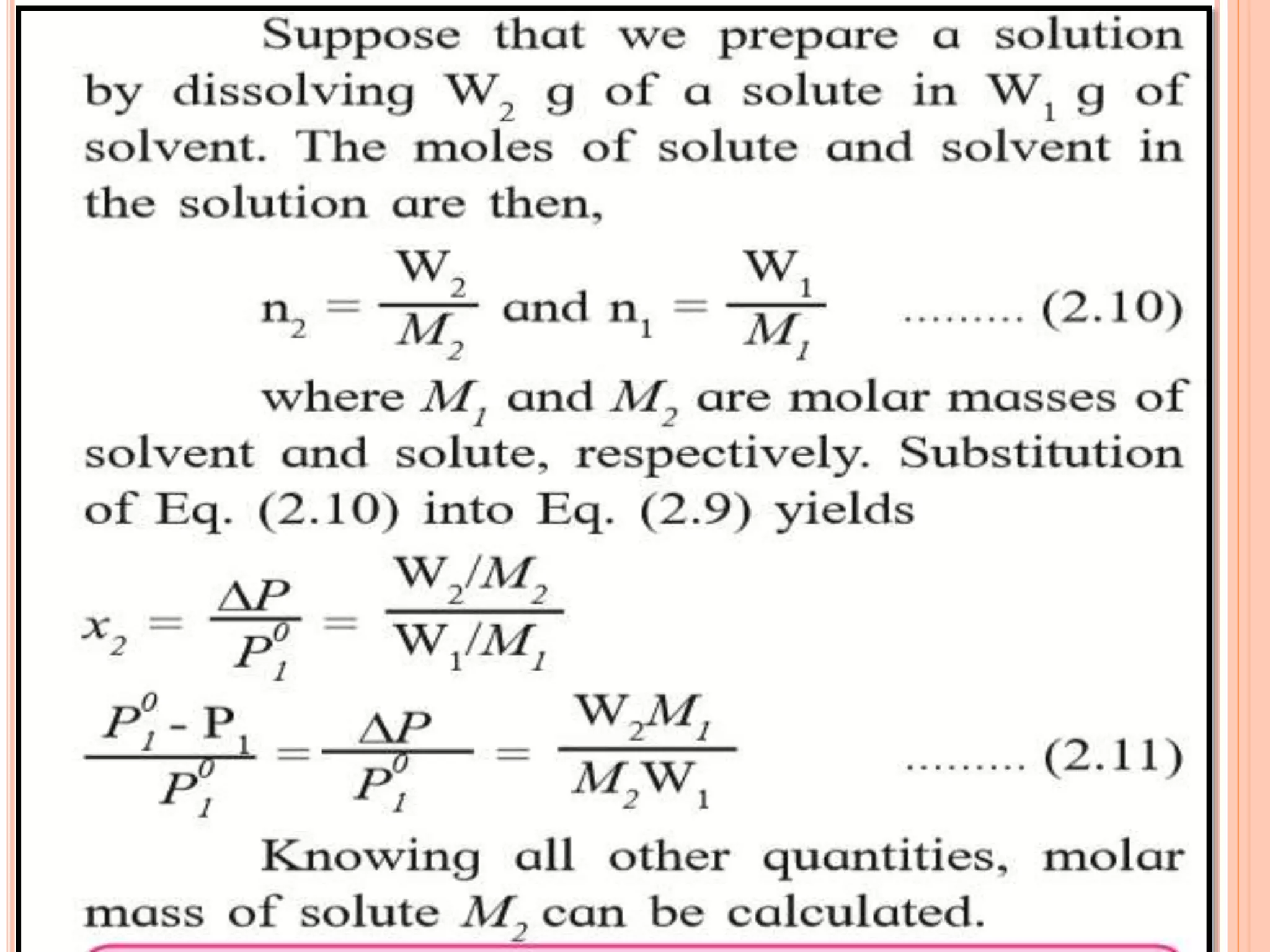
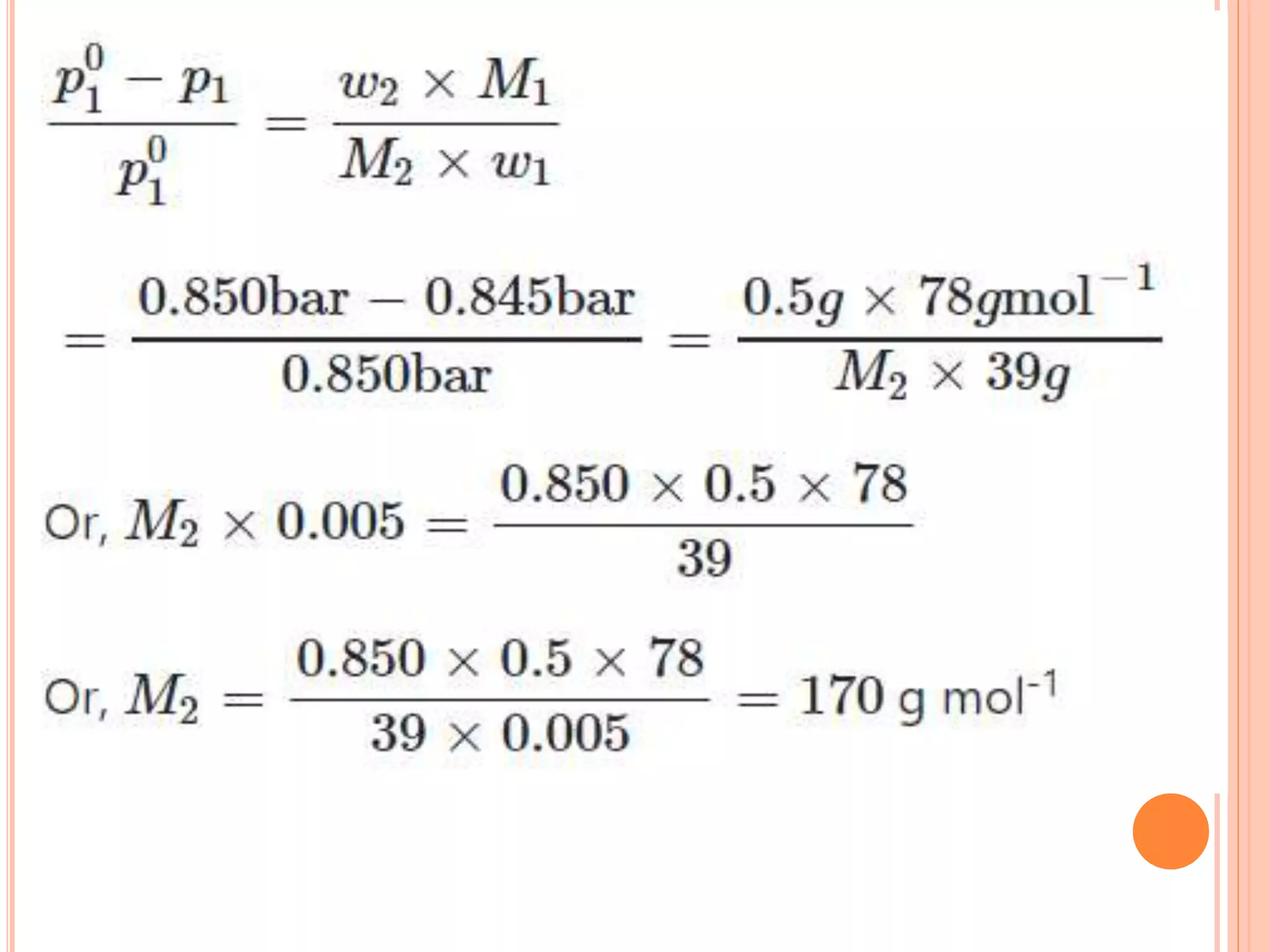
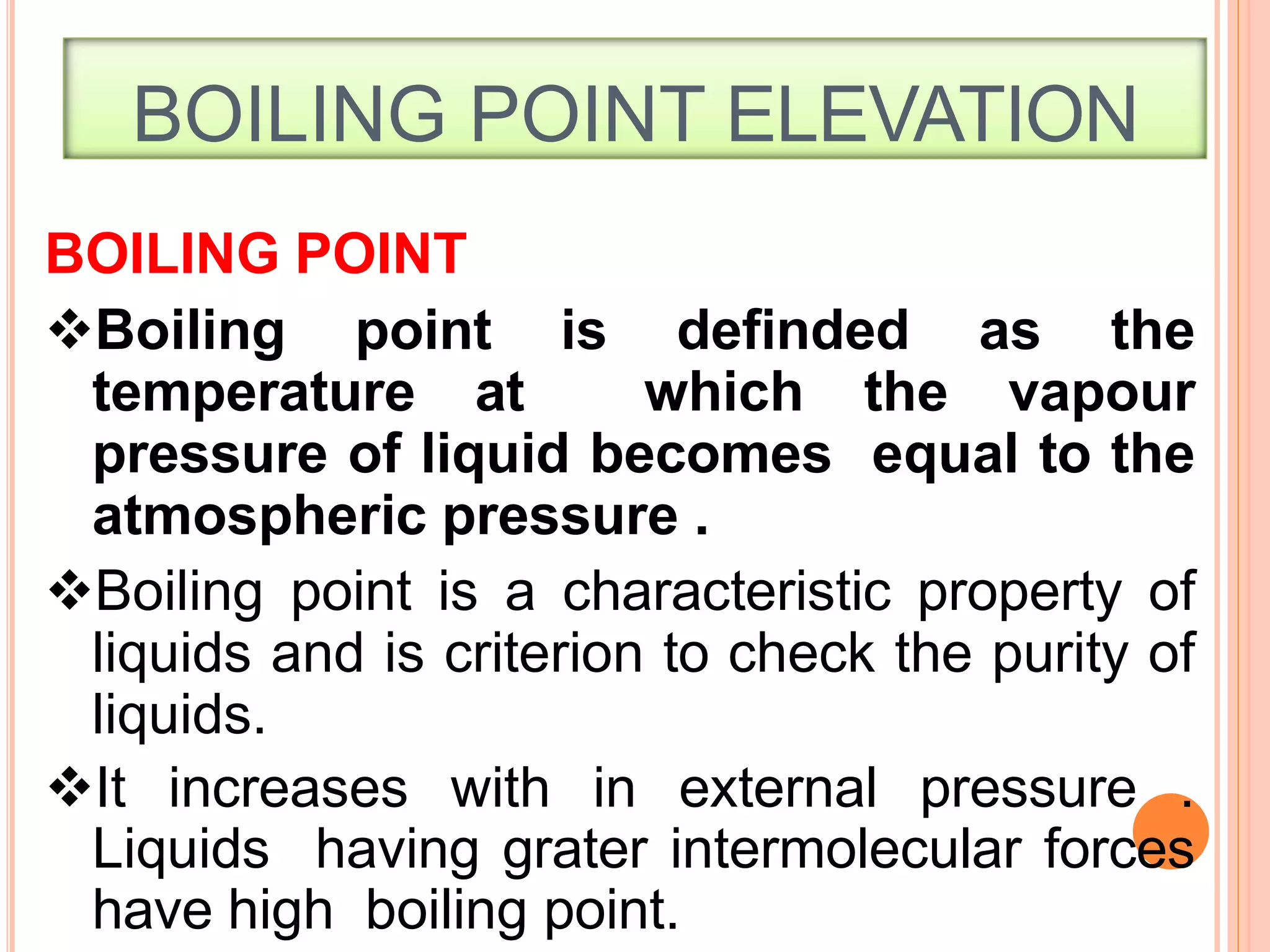
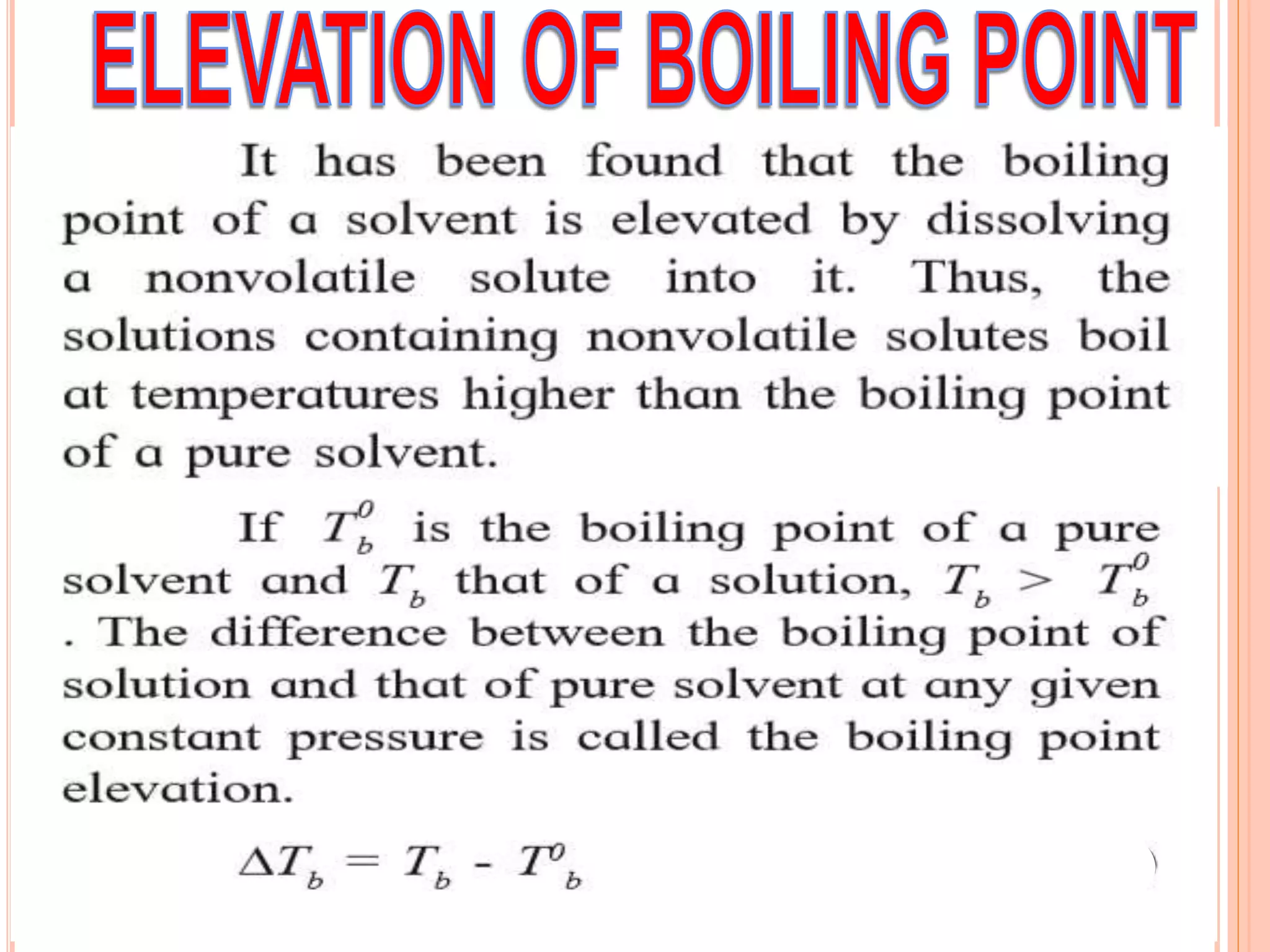
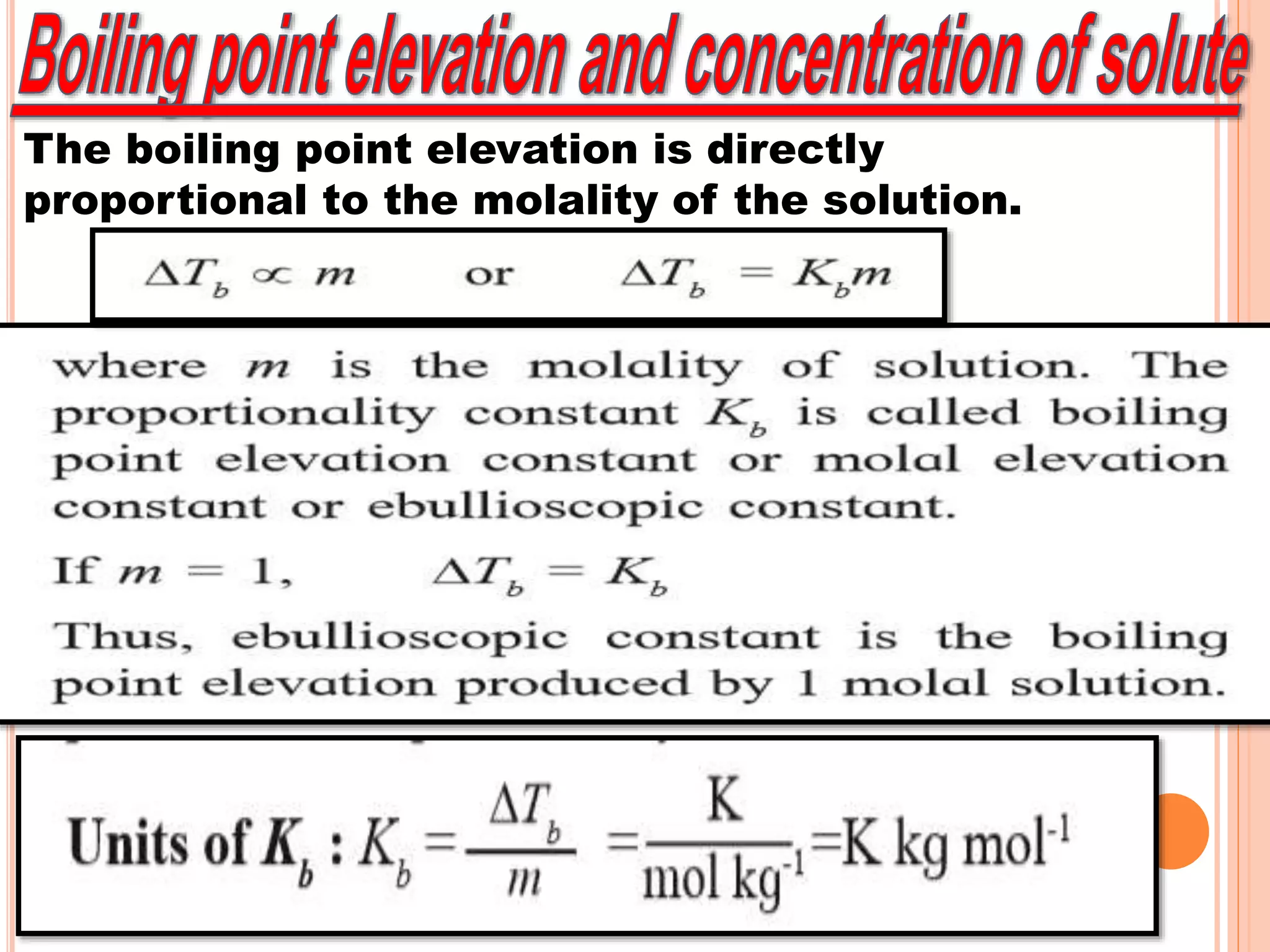
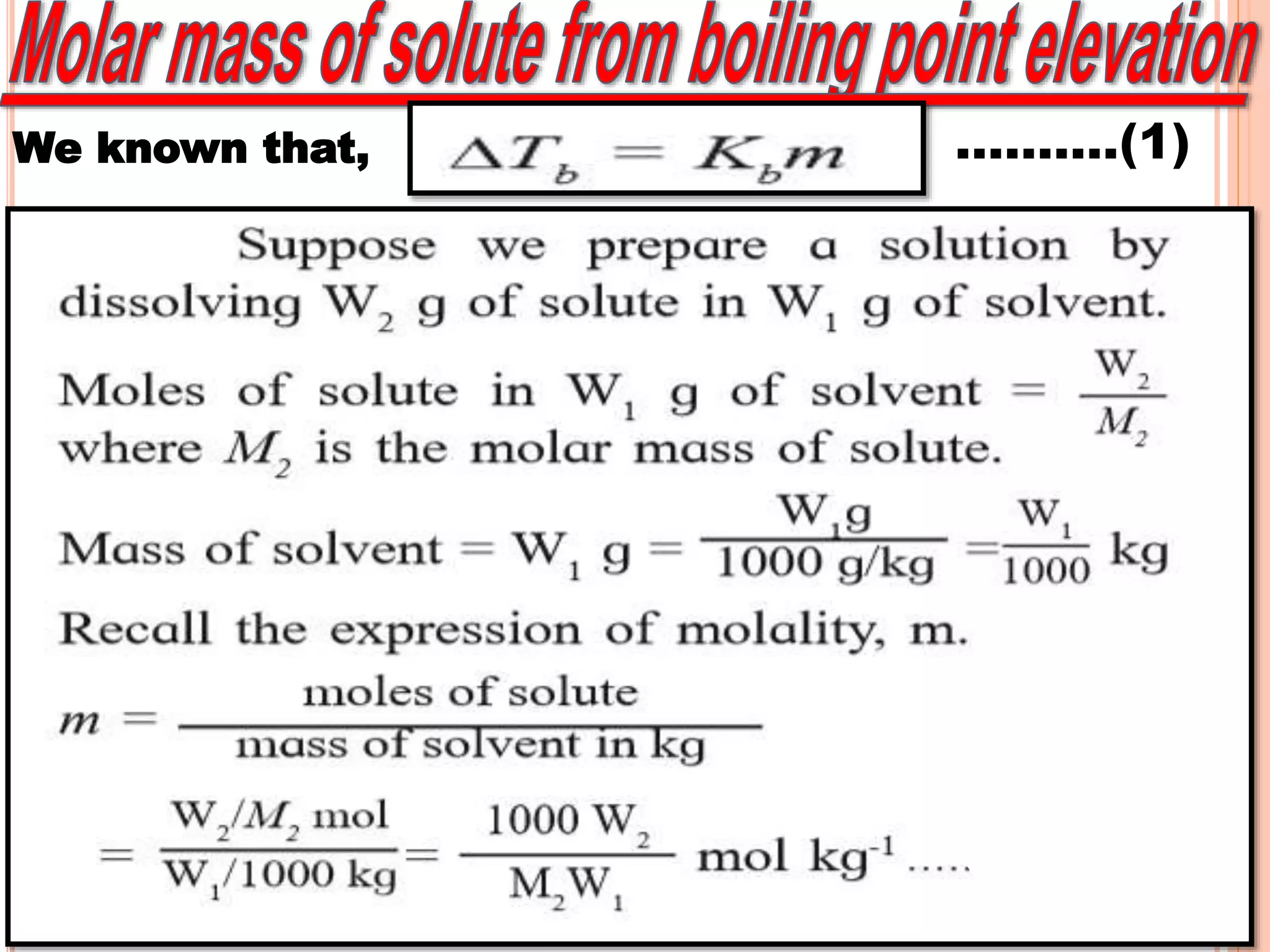
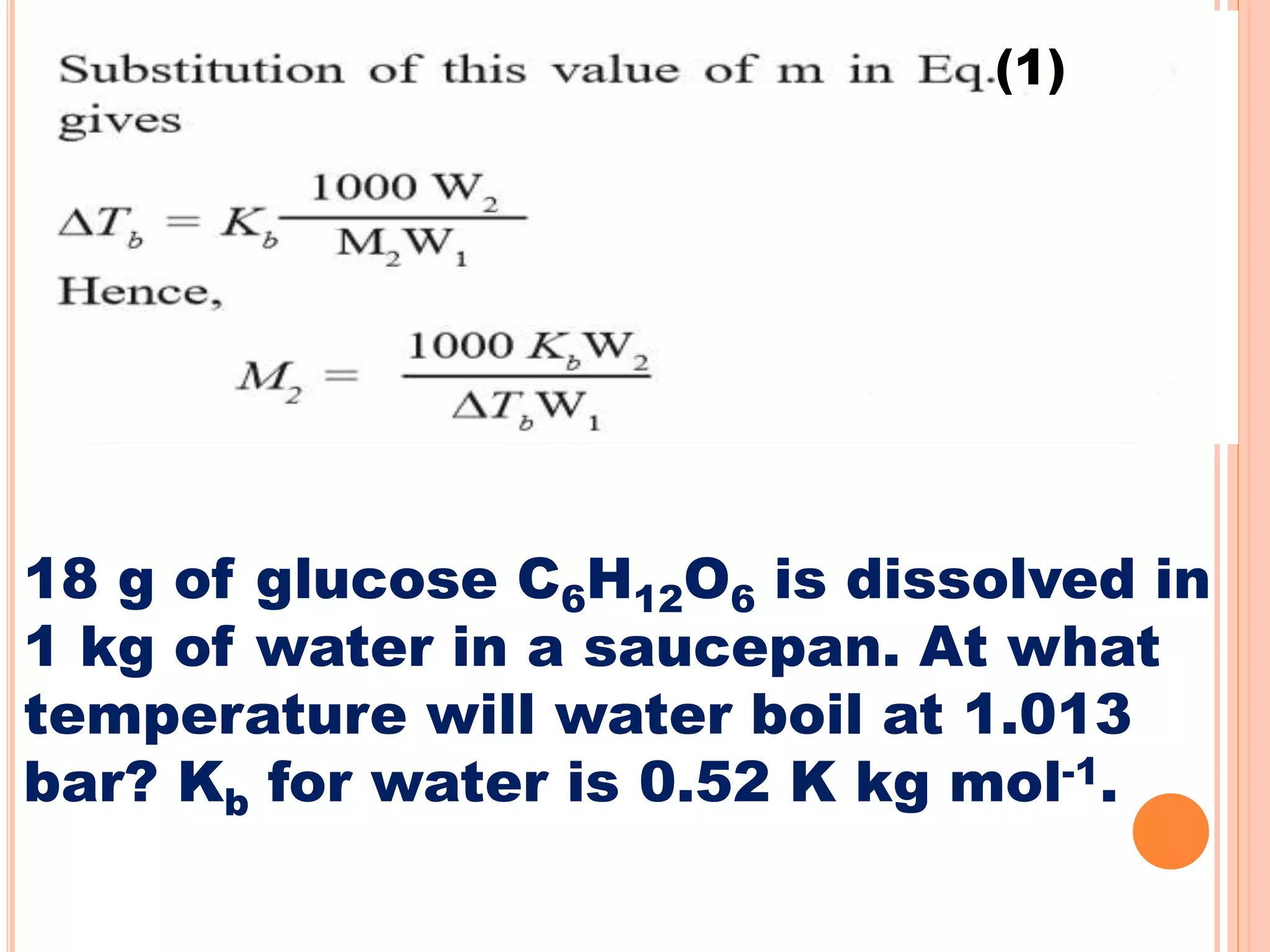
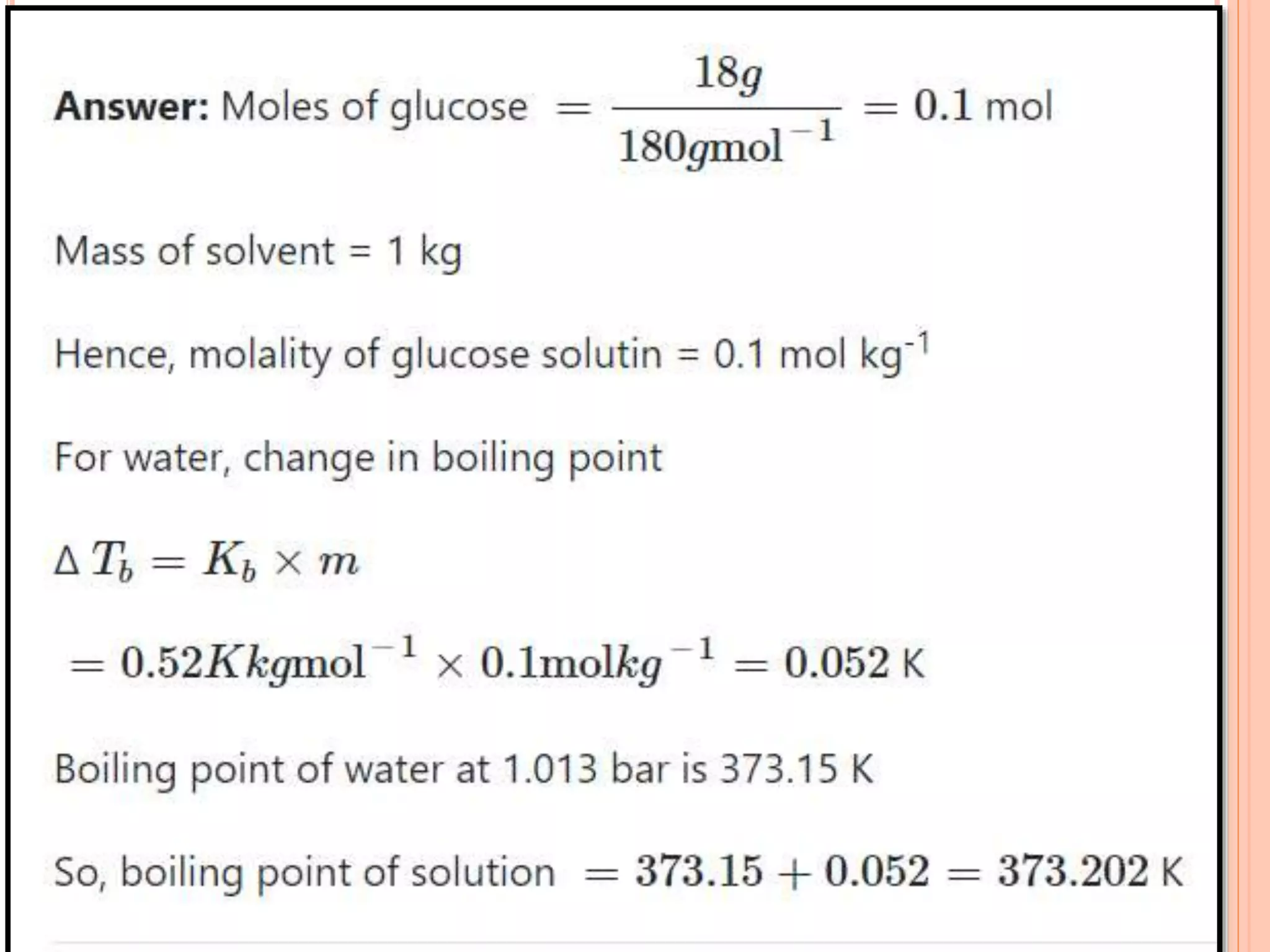
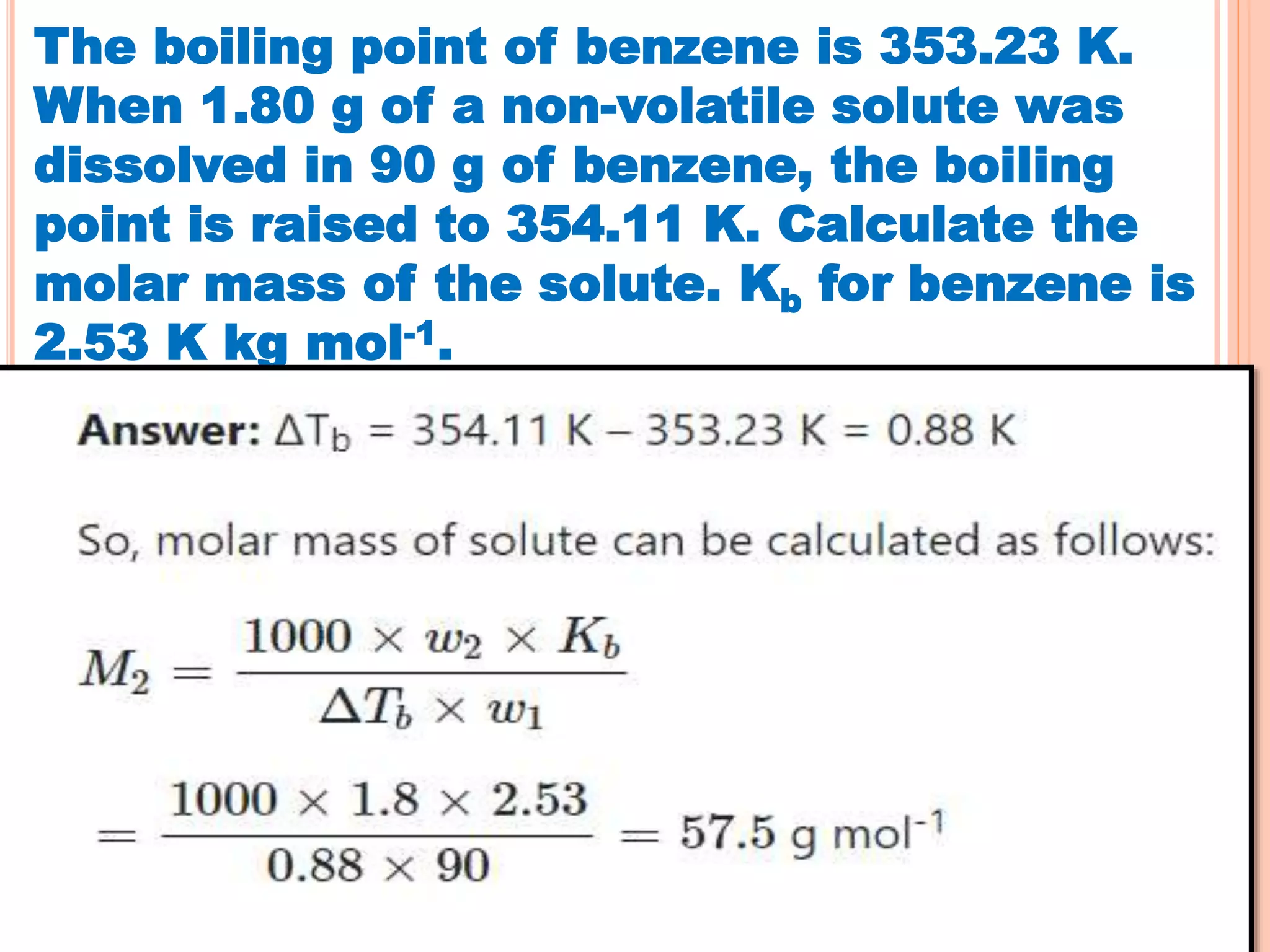
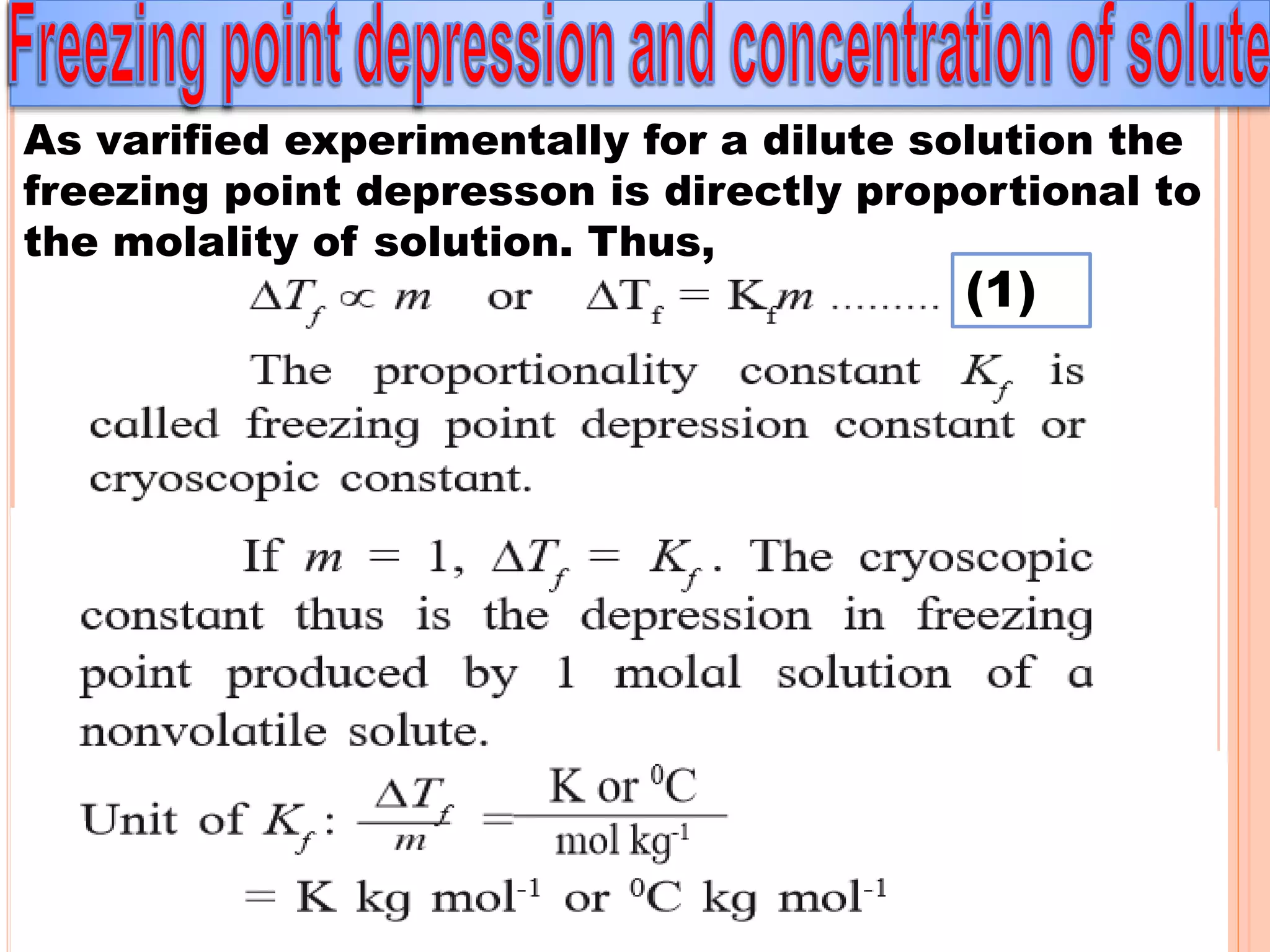
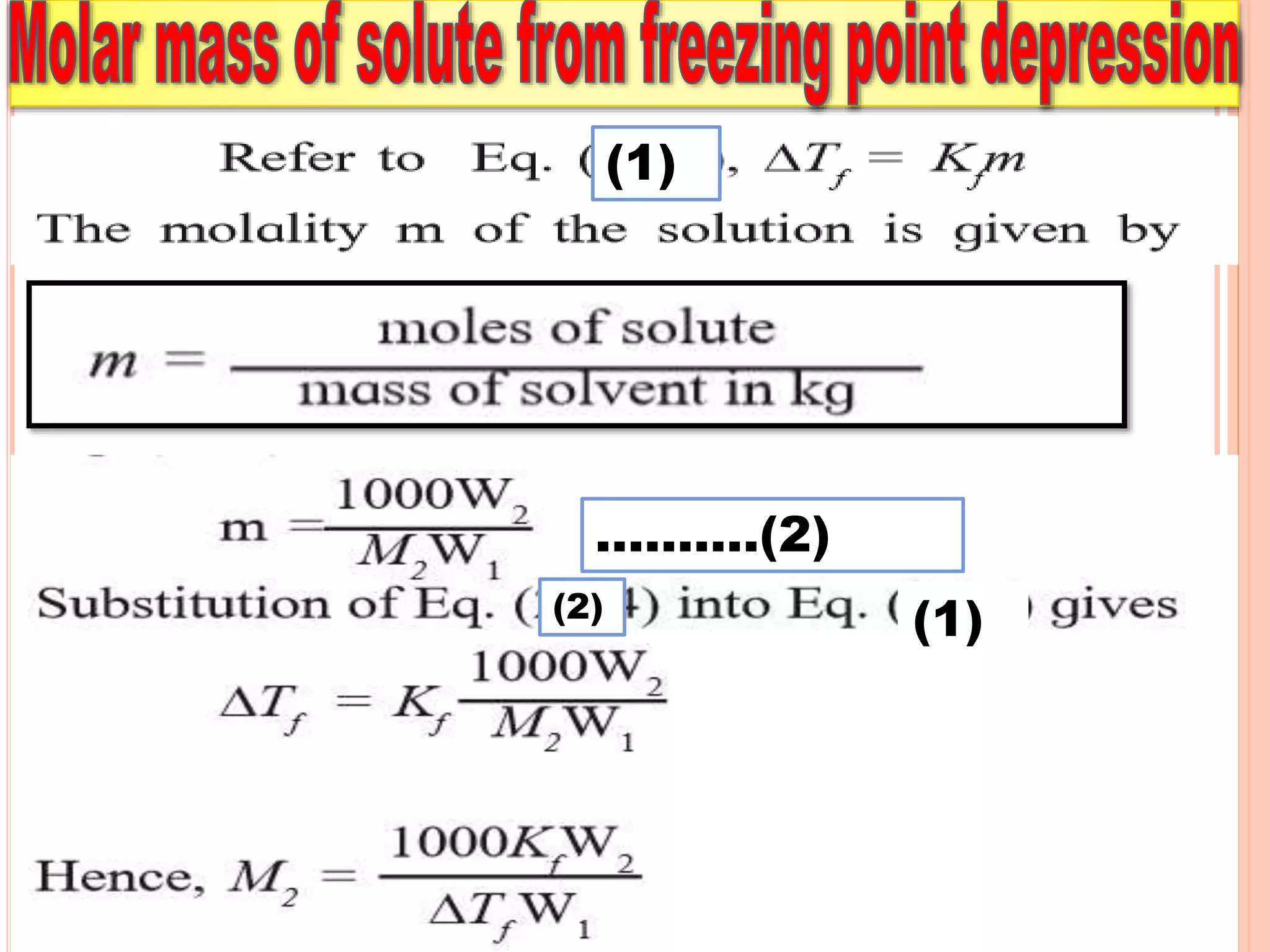
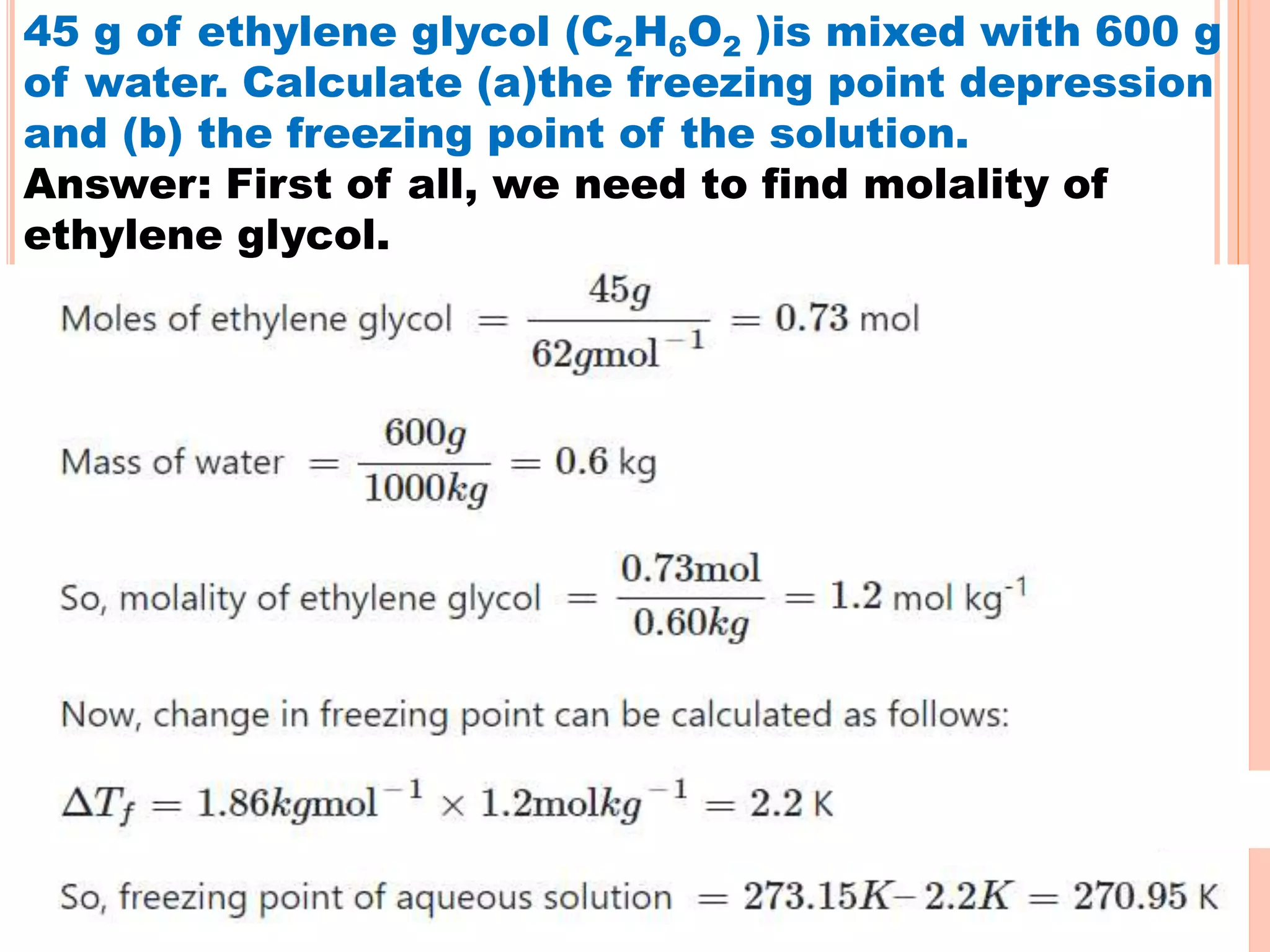
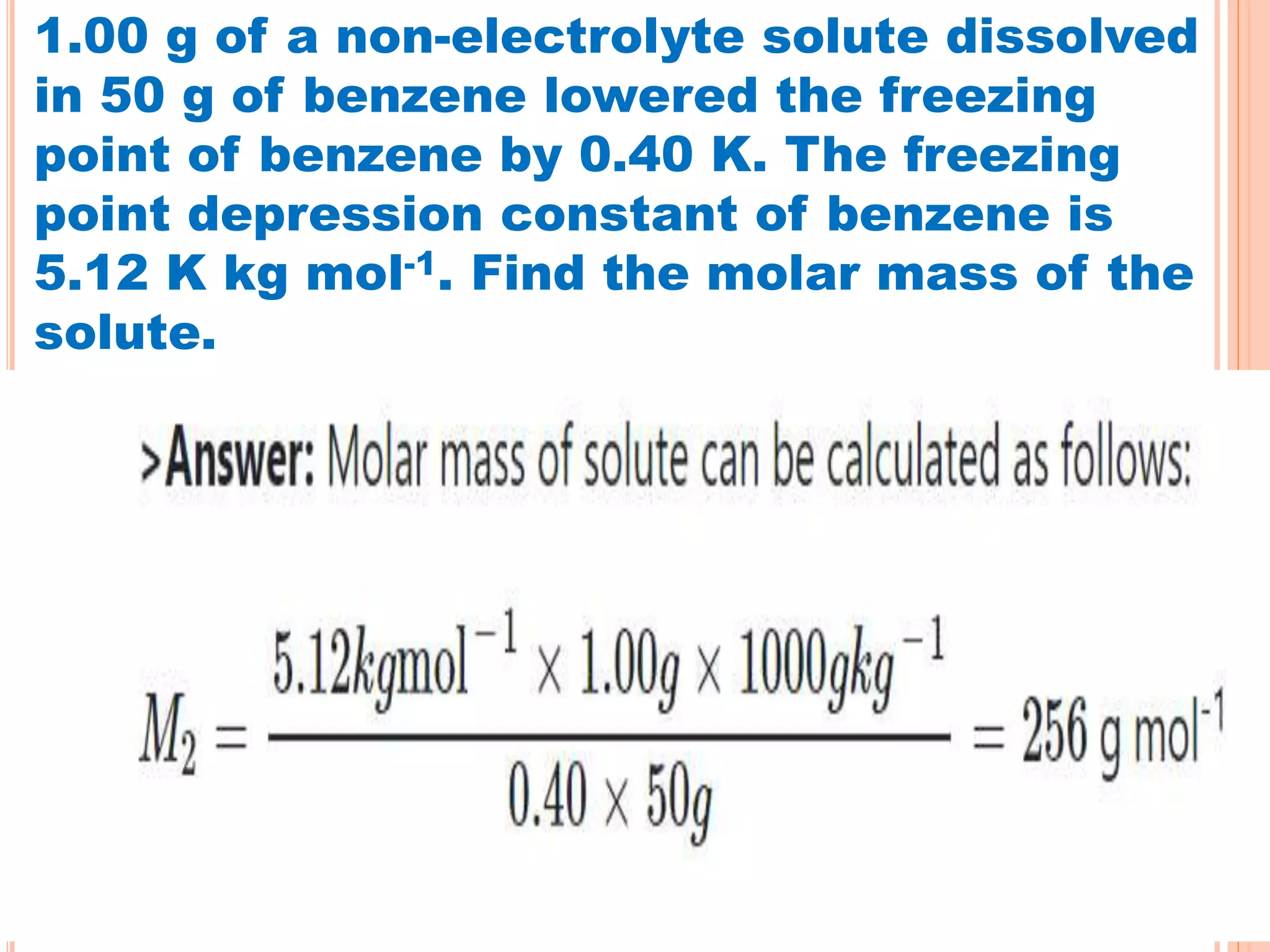
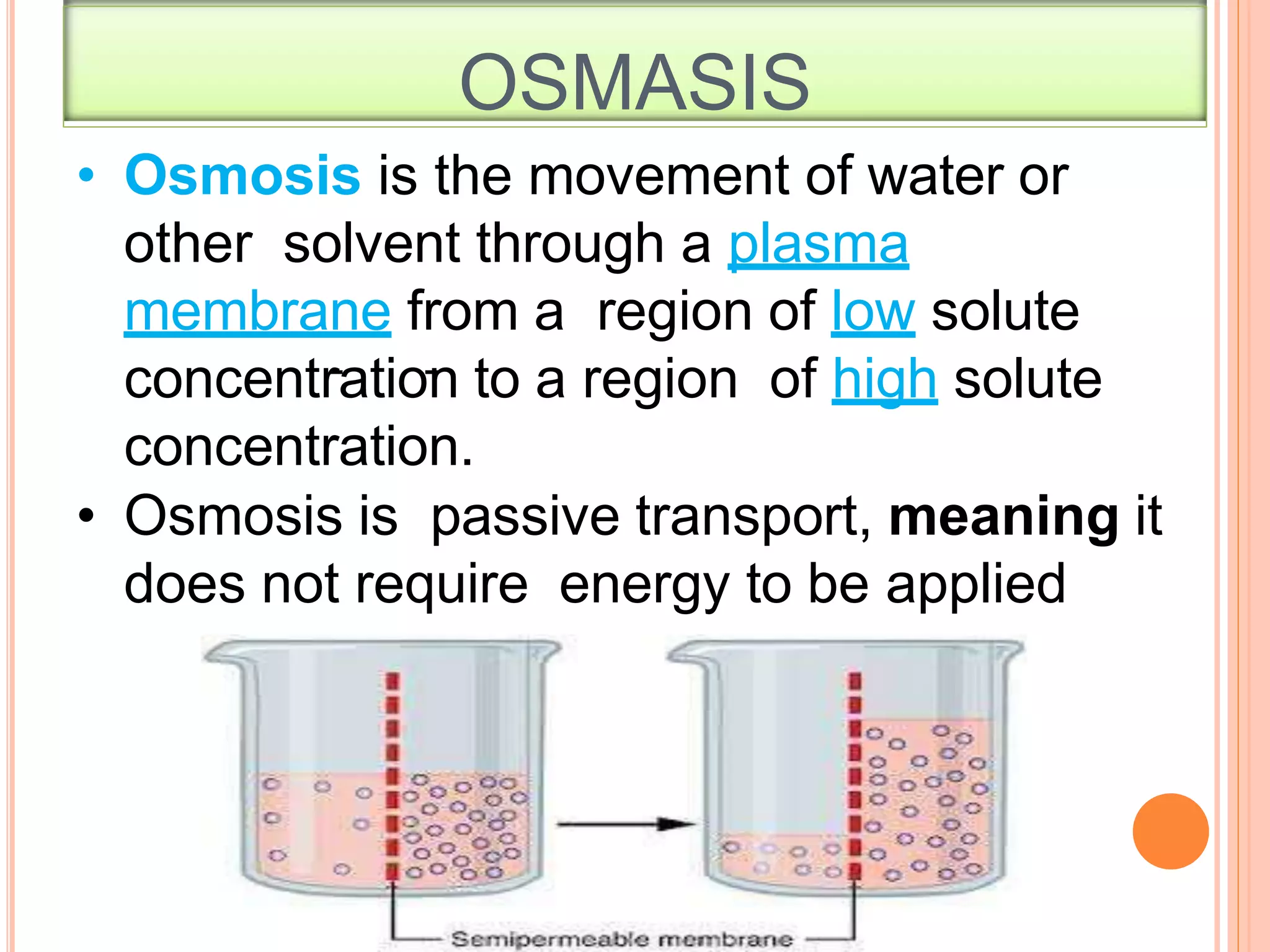
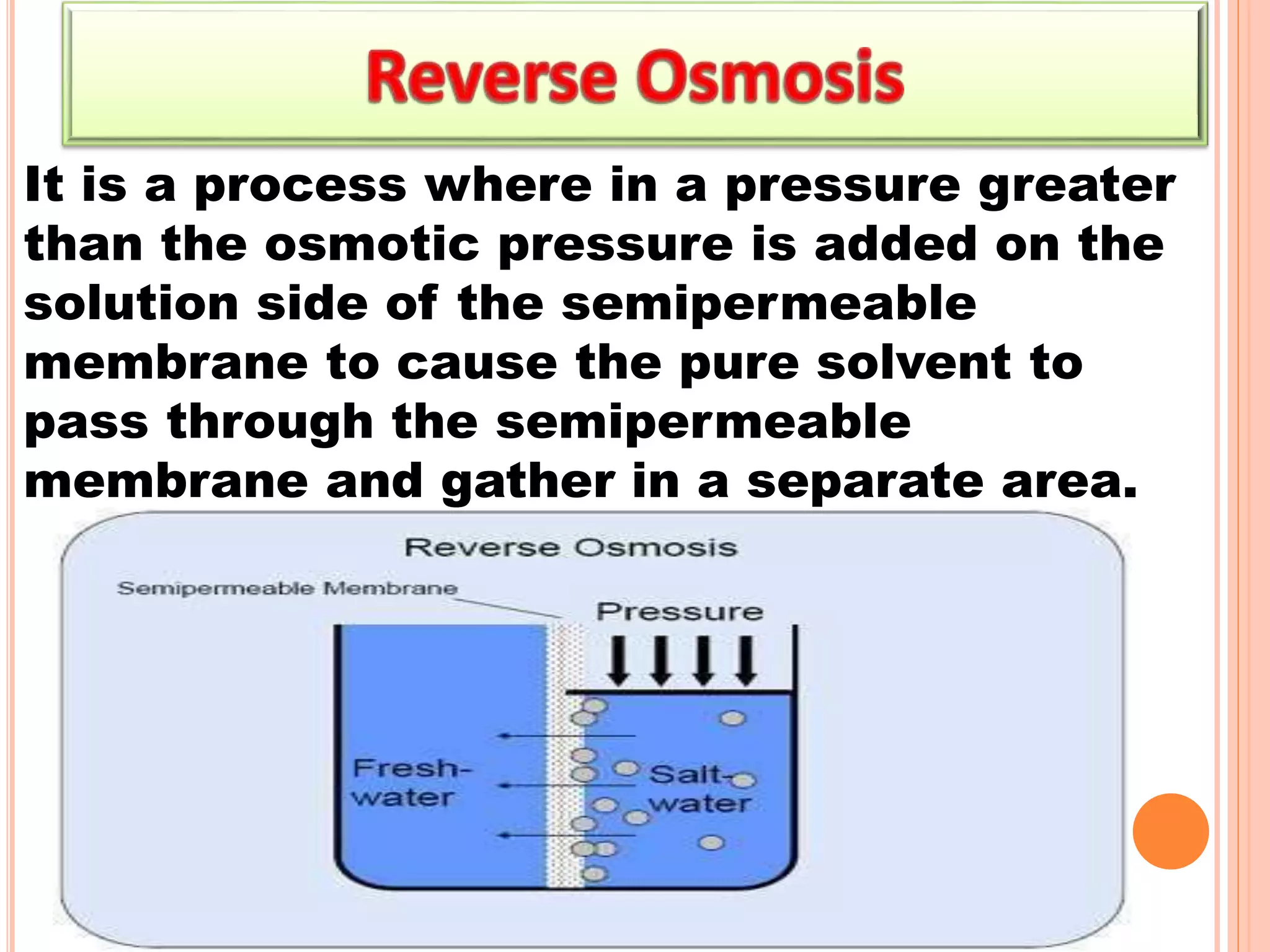
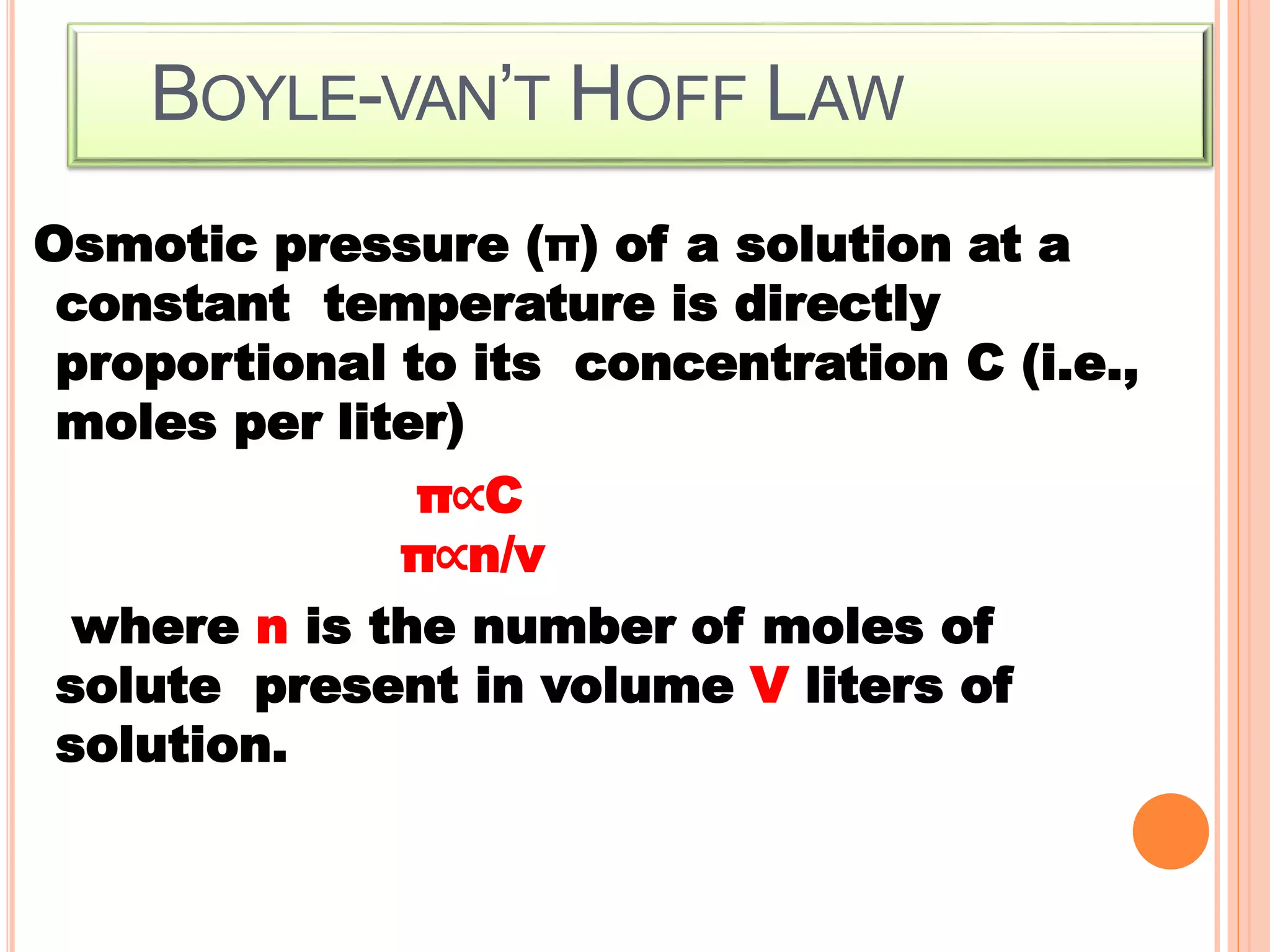
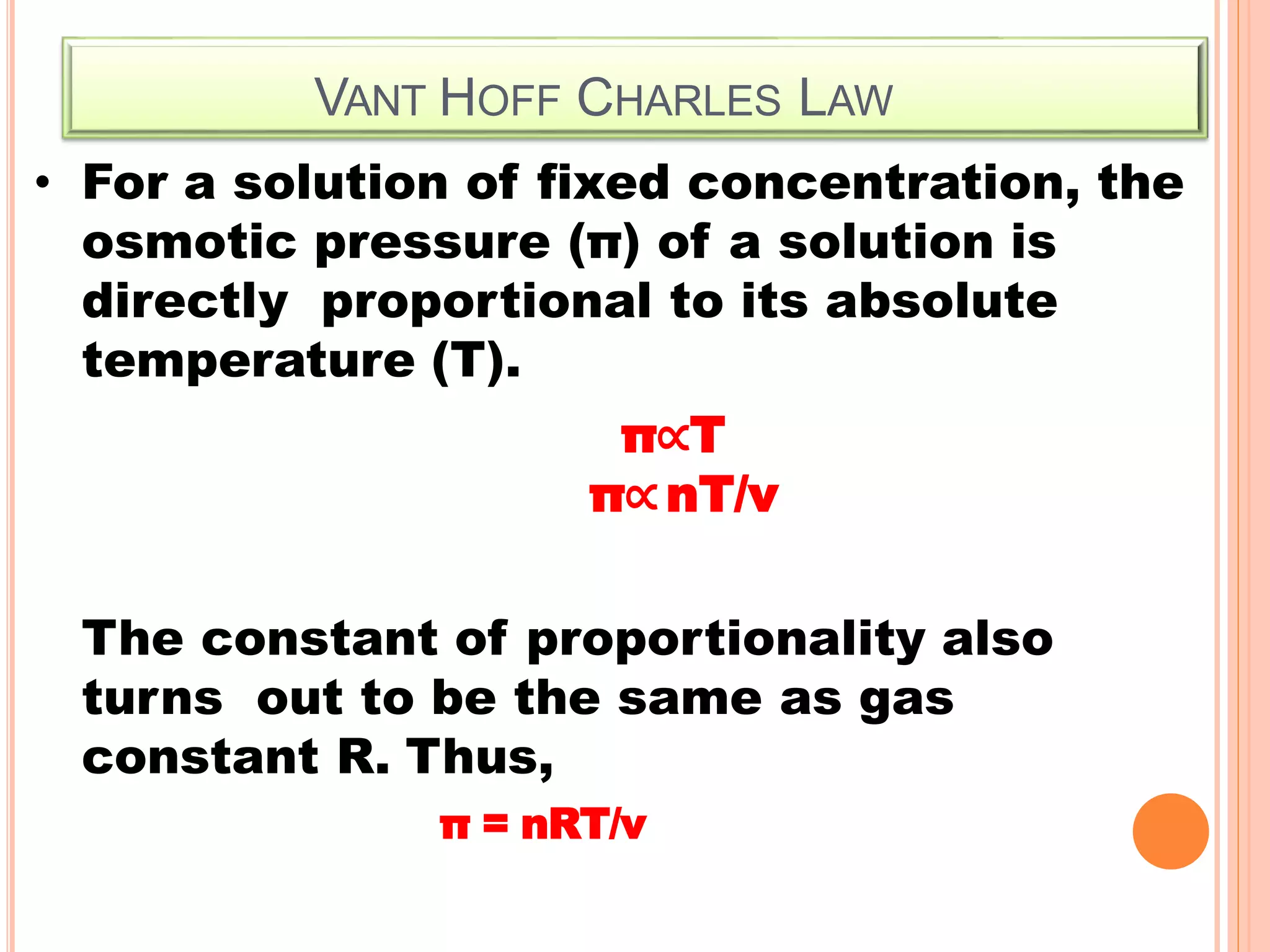
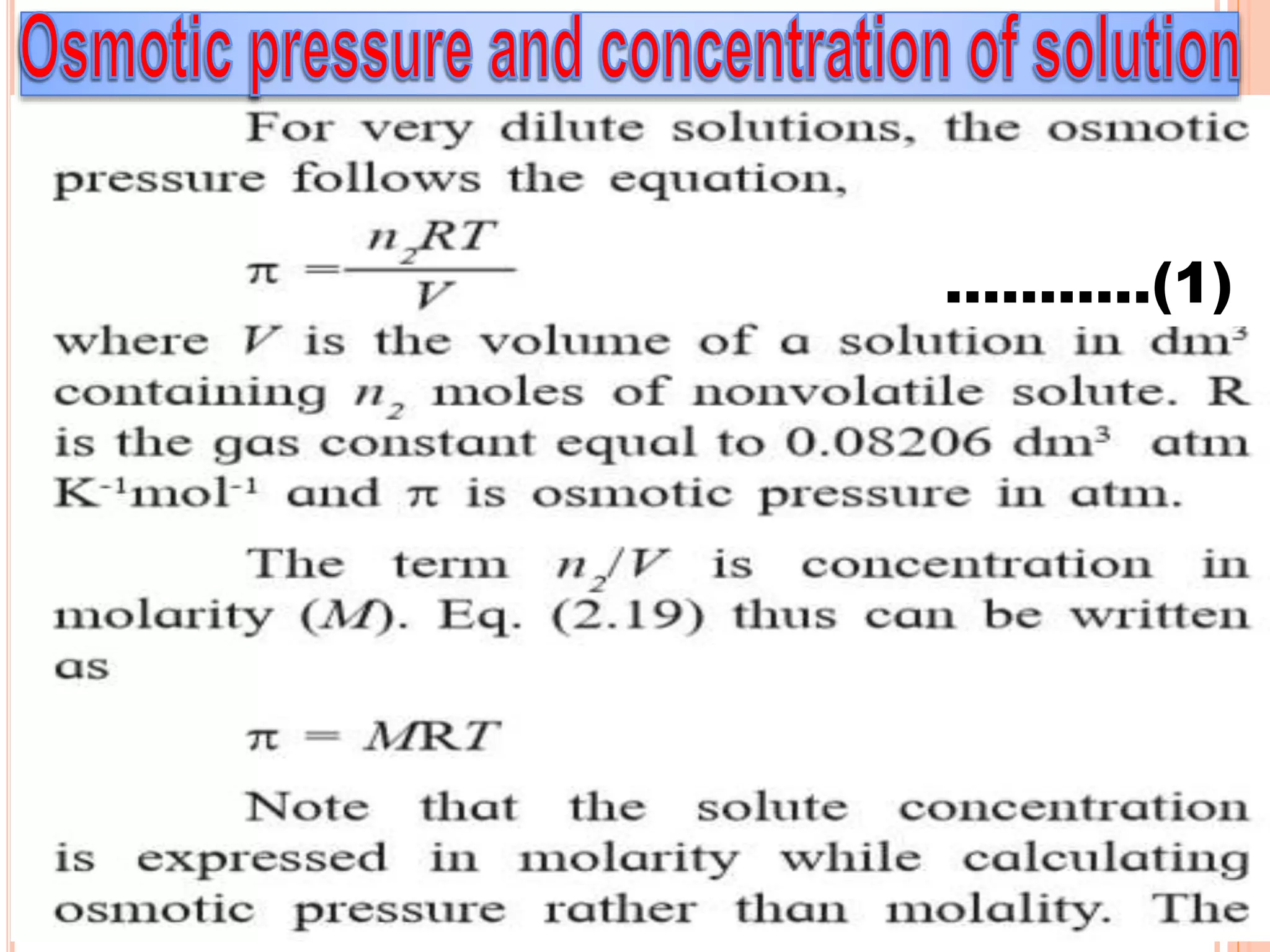
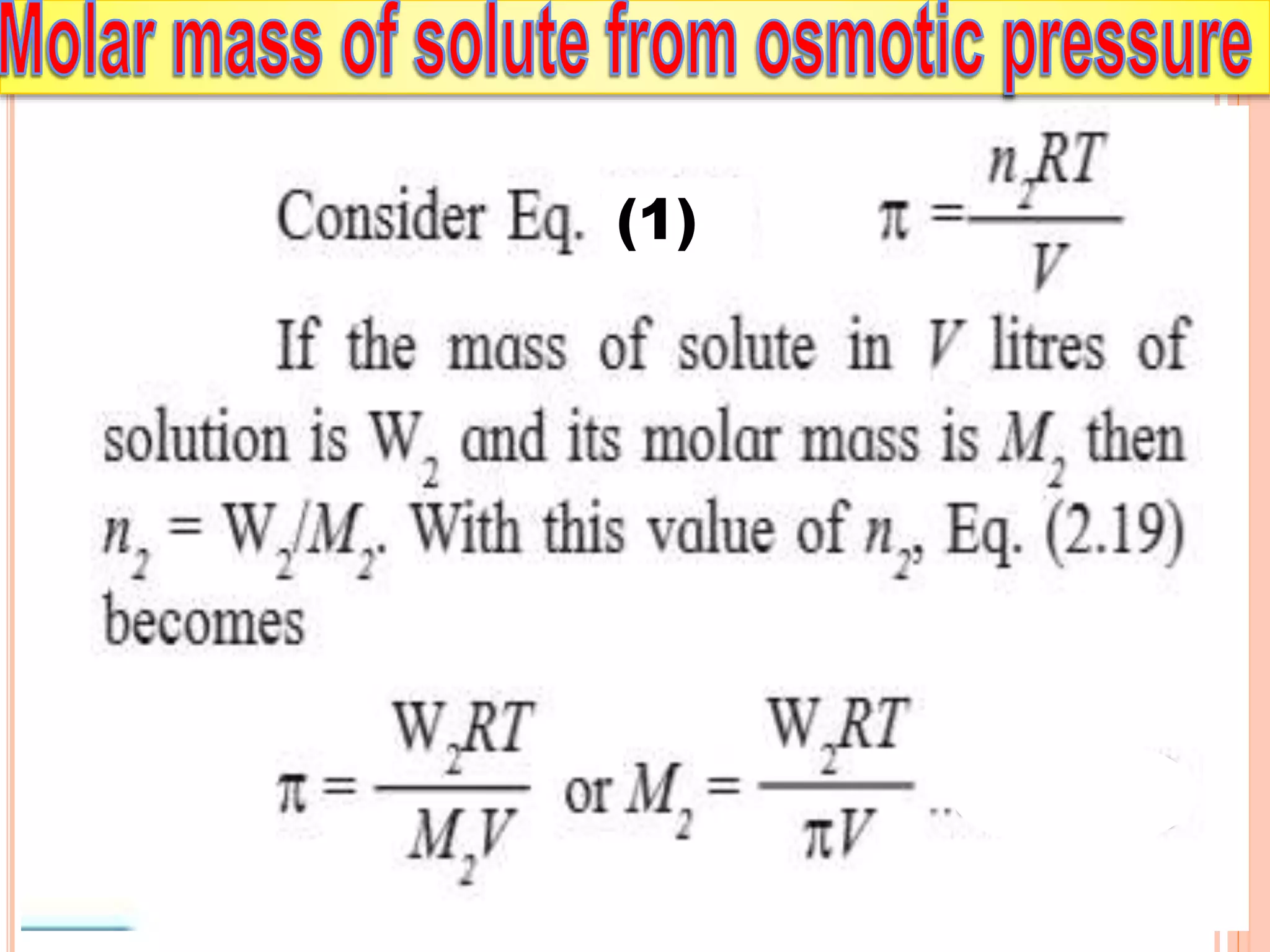
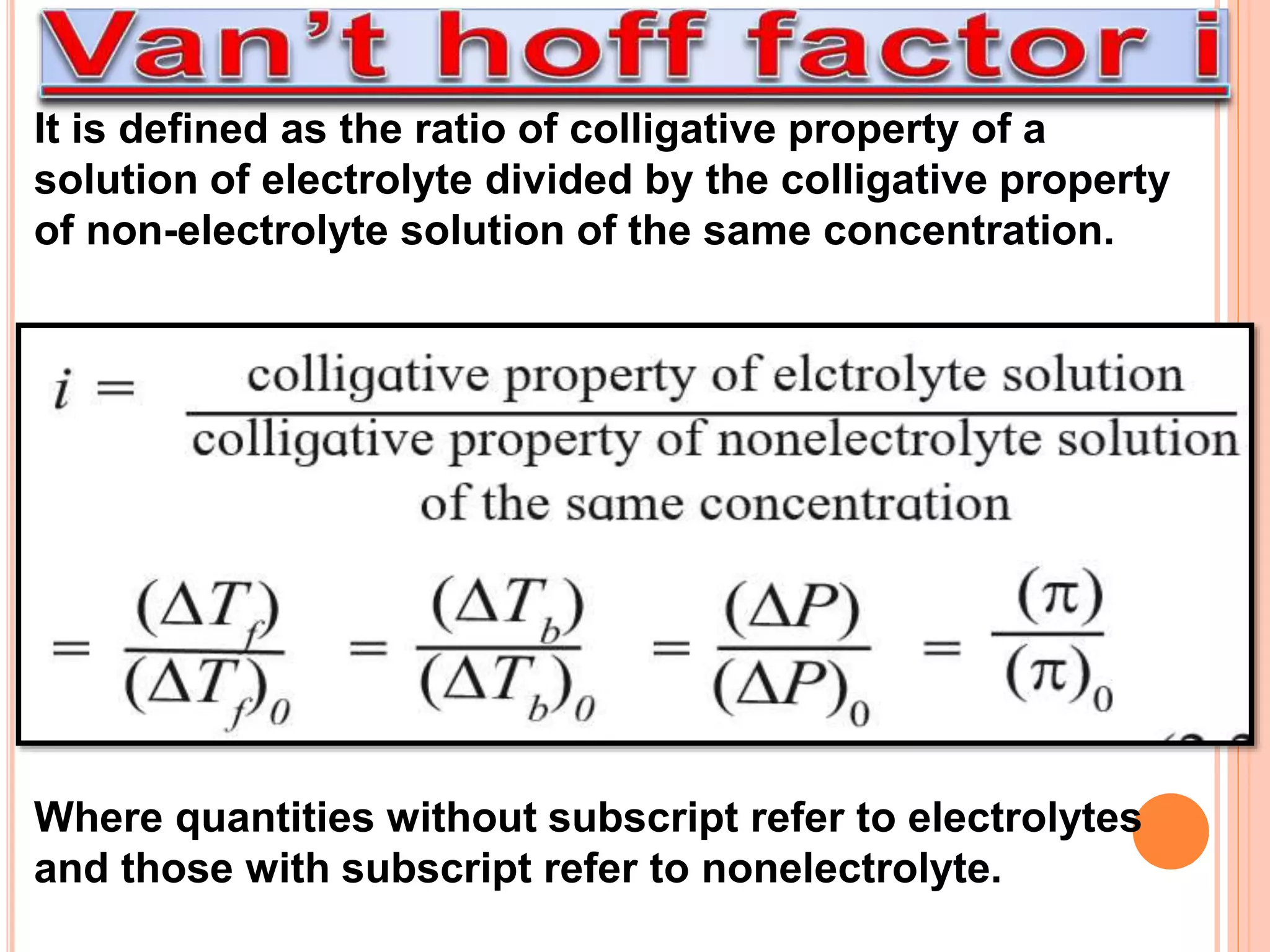
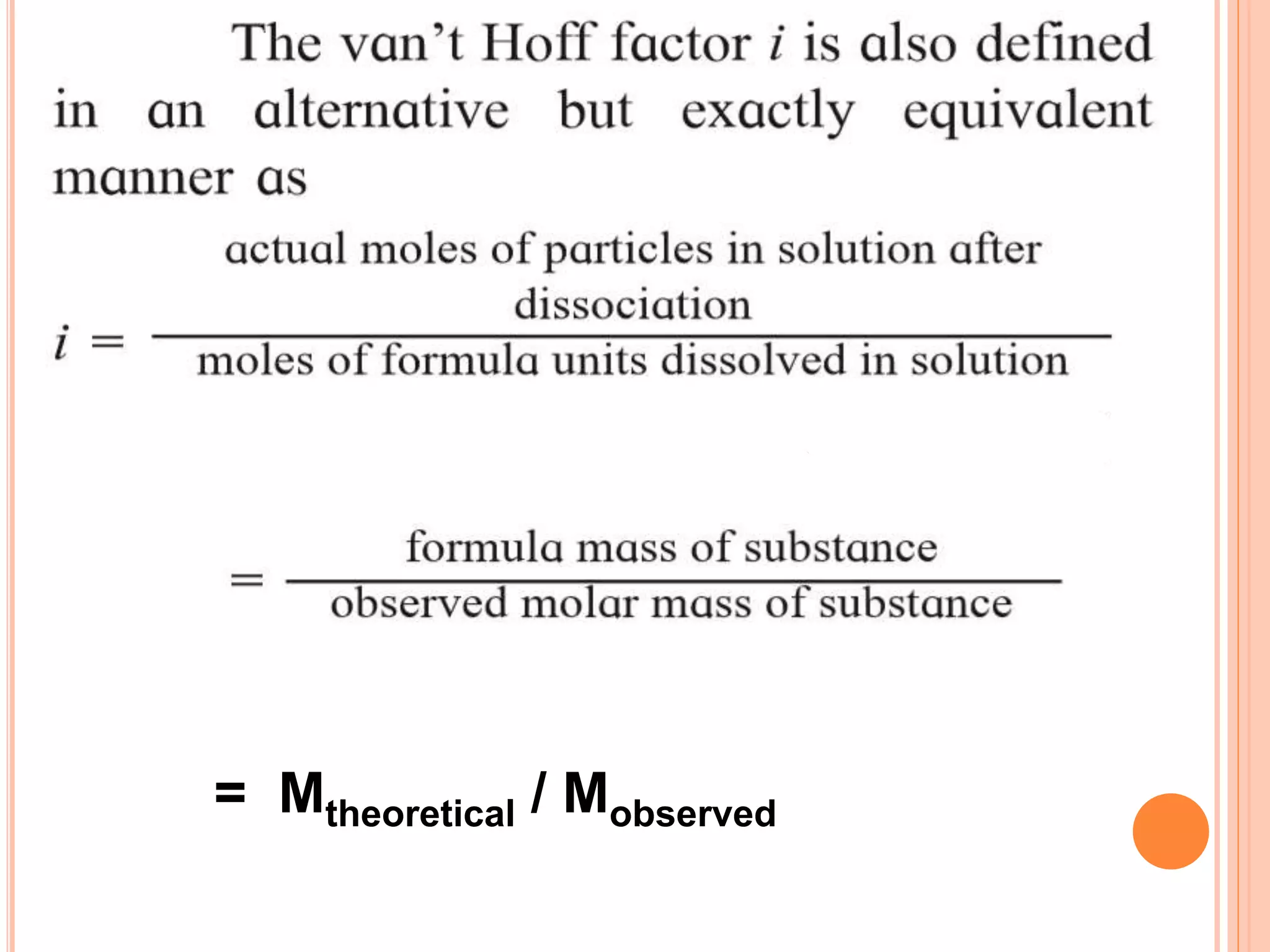
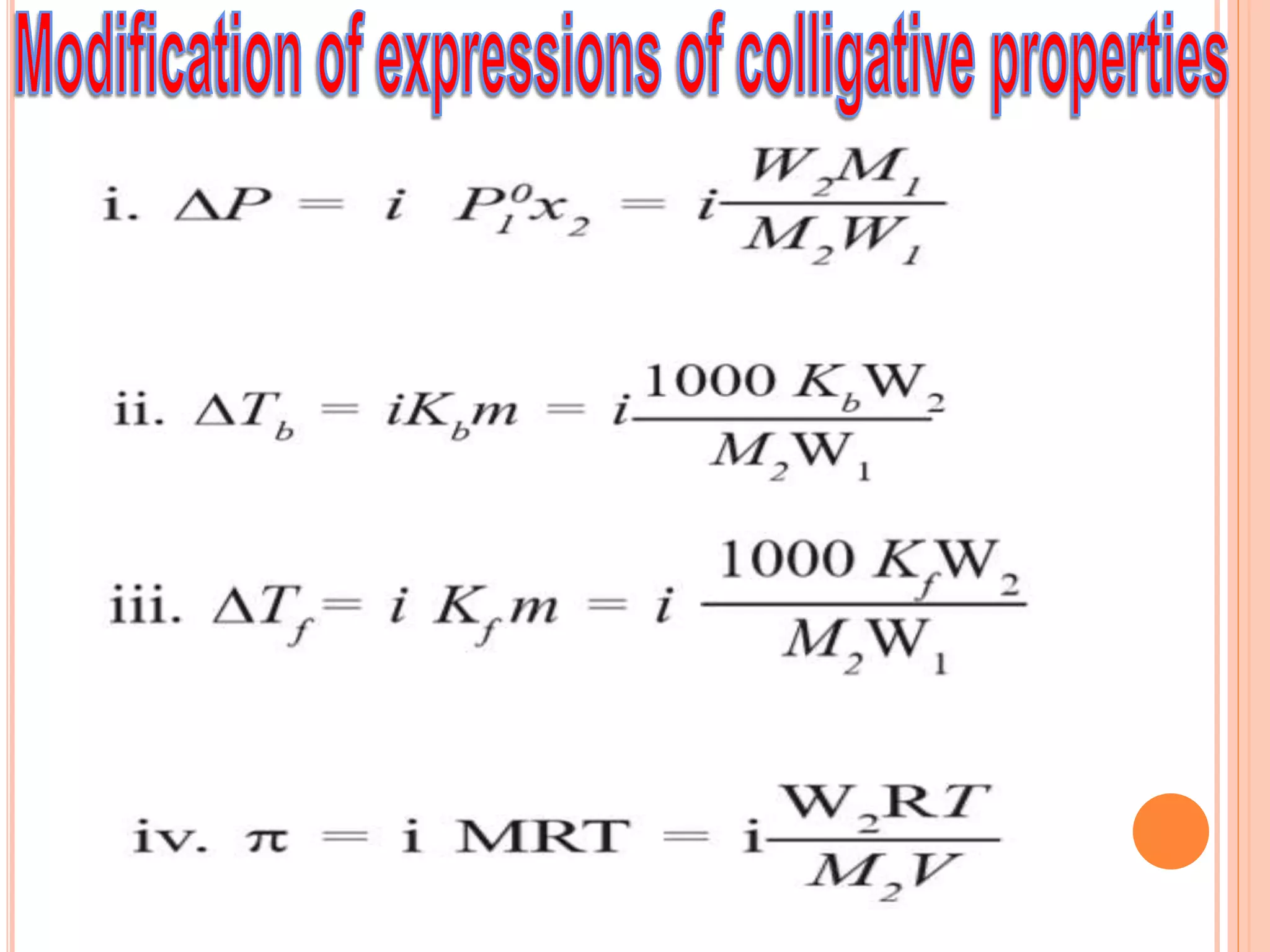
- Colligative properties that depend only on the number of solute particles such as vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.