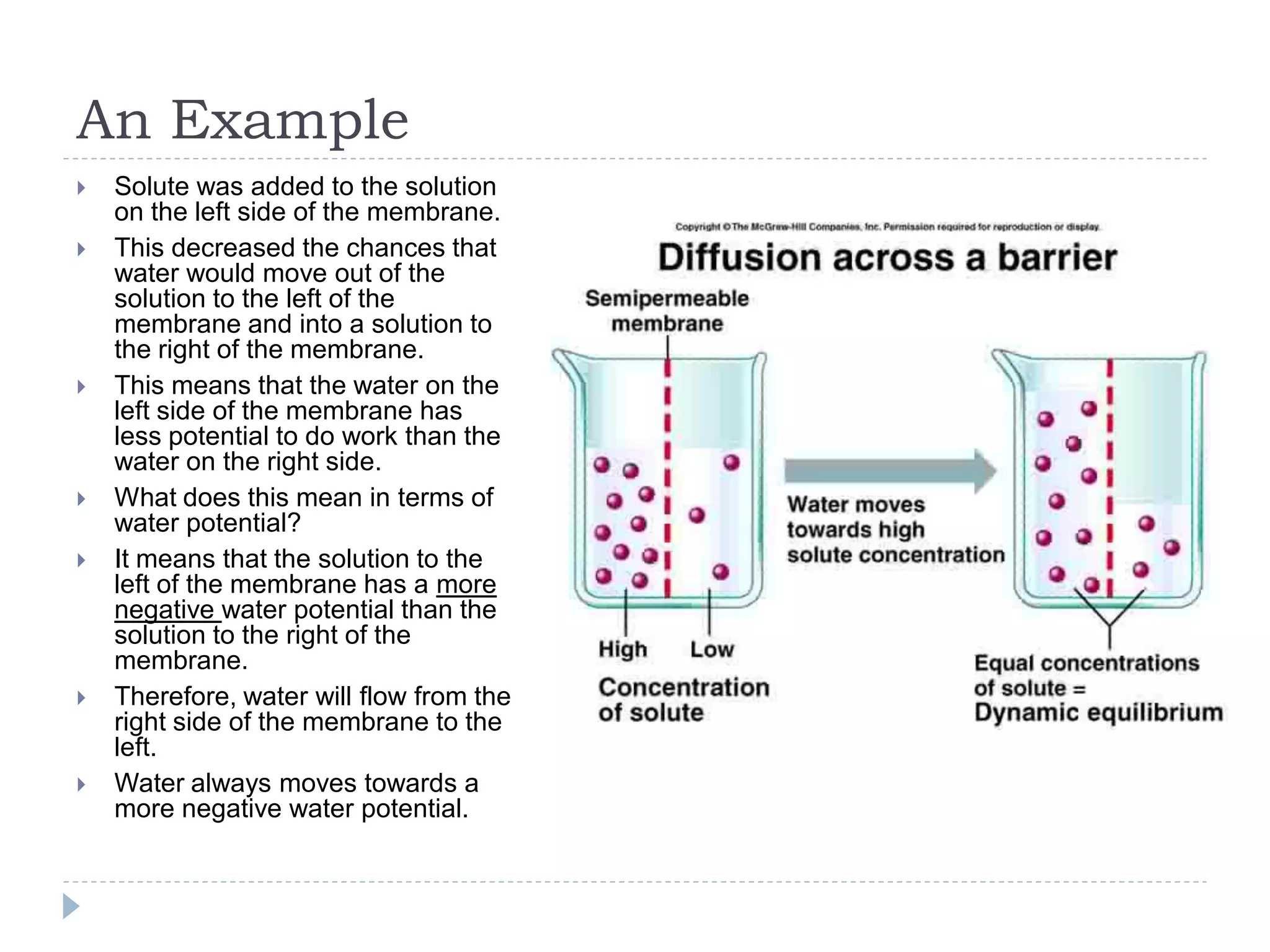
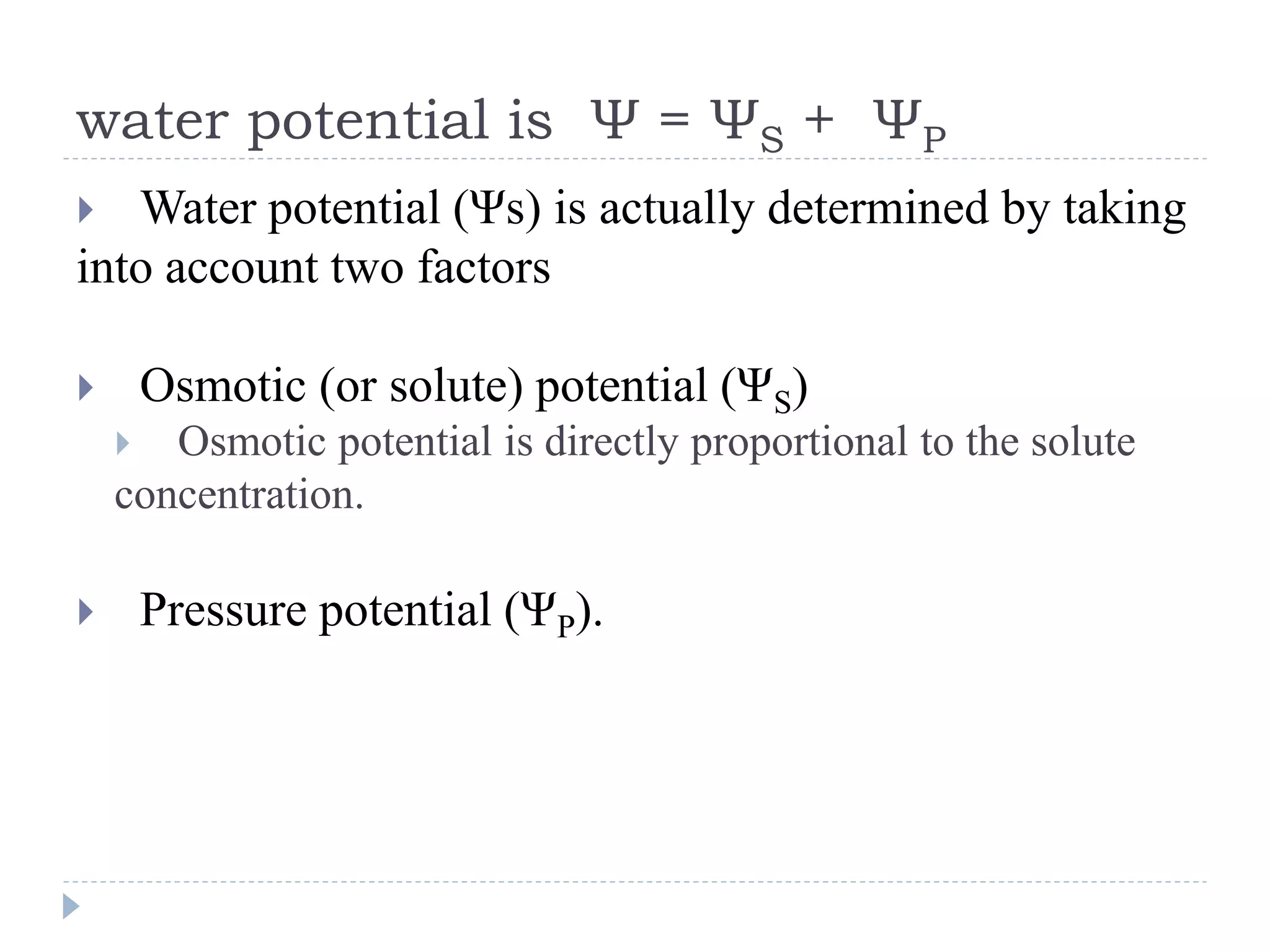
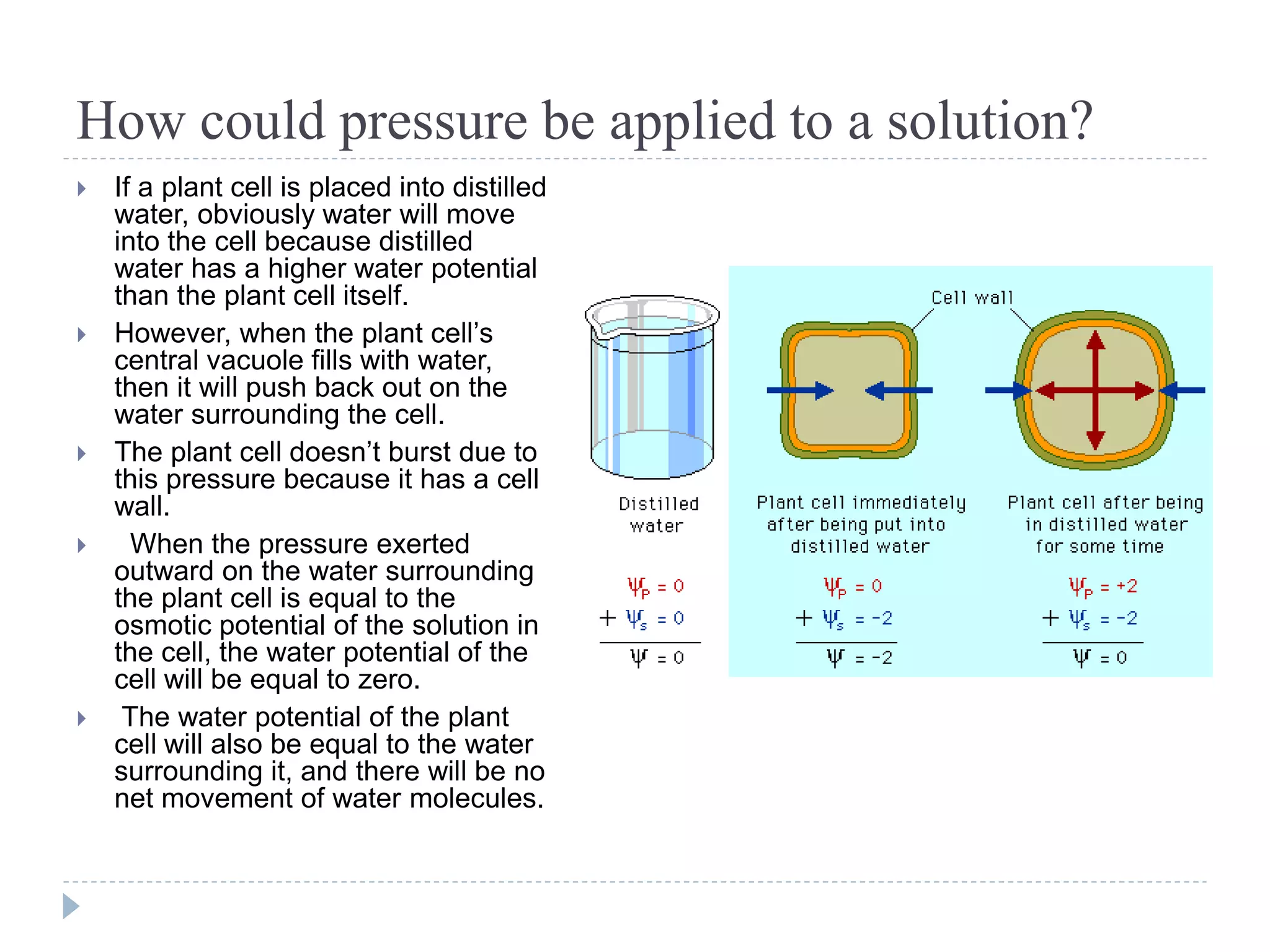
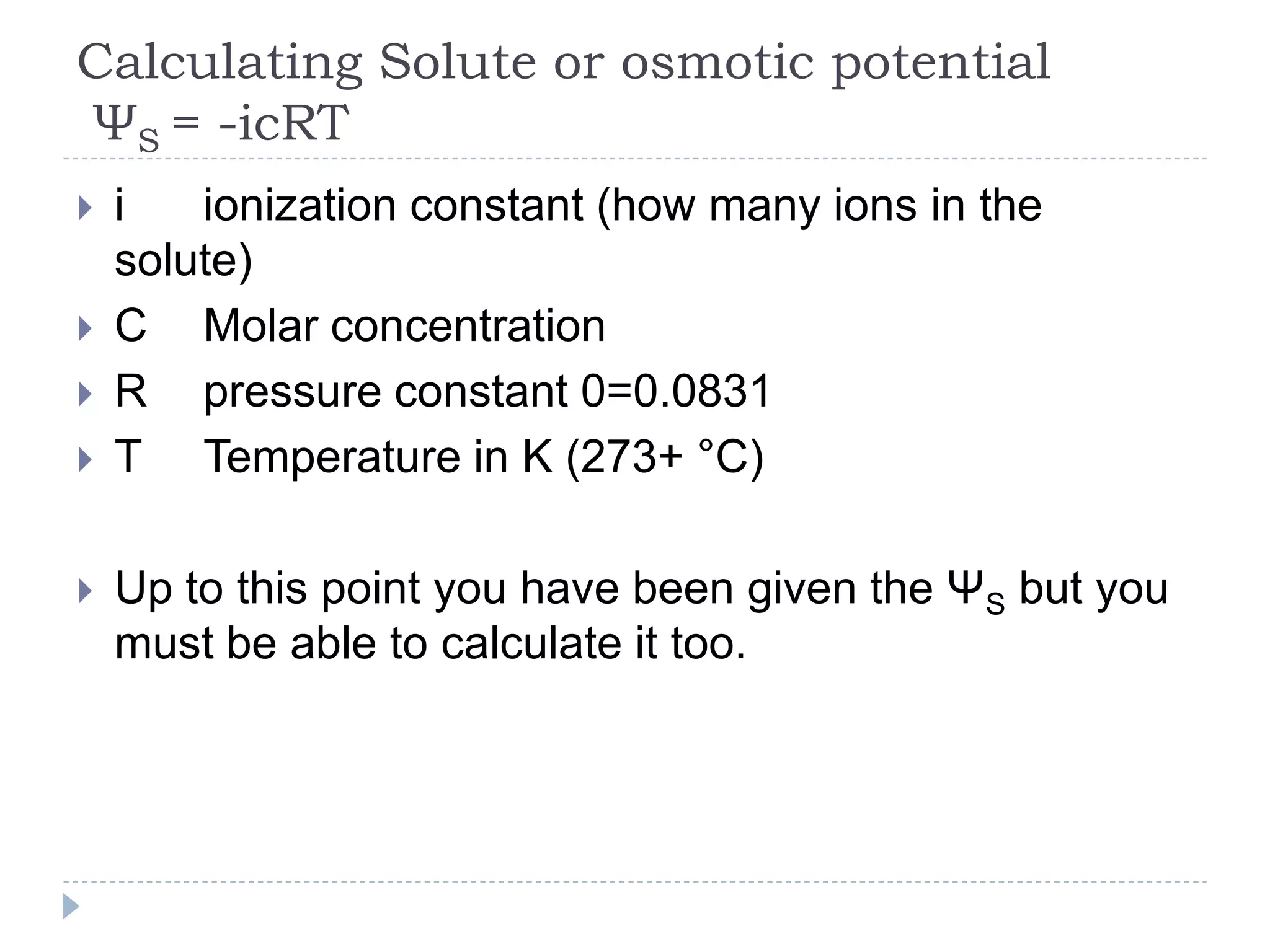
Water potential (Ψ) is a measure of water's ability to do work and move. It is determined by solute potential (Ψs), which decreases as solute concentration increases, making water less likely to move, and pressure potential (Ψp). In solutions, water moves from areas of higher to lower water potential. Adding solute decreases a solution's water potential by lowering Ψs. When a plant cell is placed in a solution, water enters until pressure builds and Ψp increases, balancing the cell's Ψs so net movement stops at equilibrium.