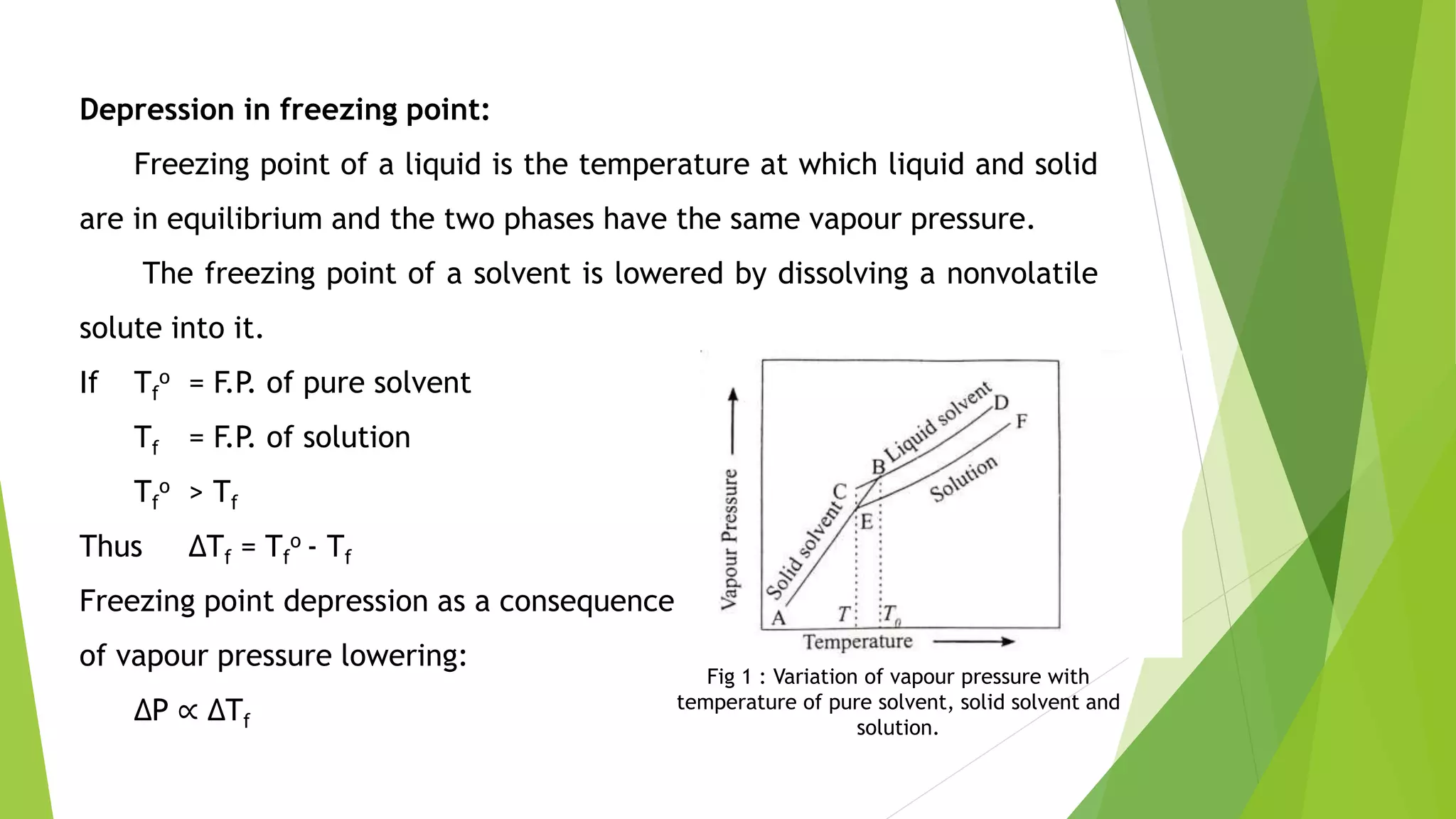
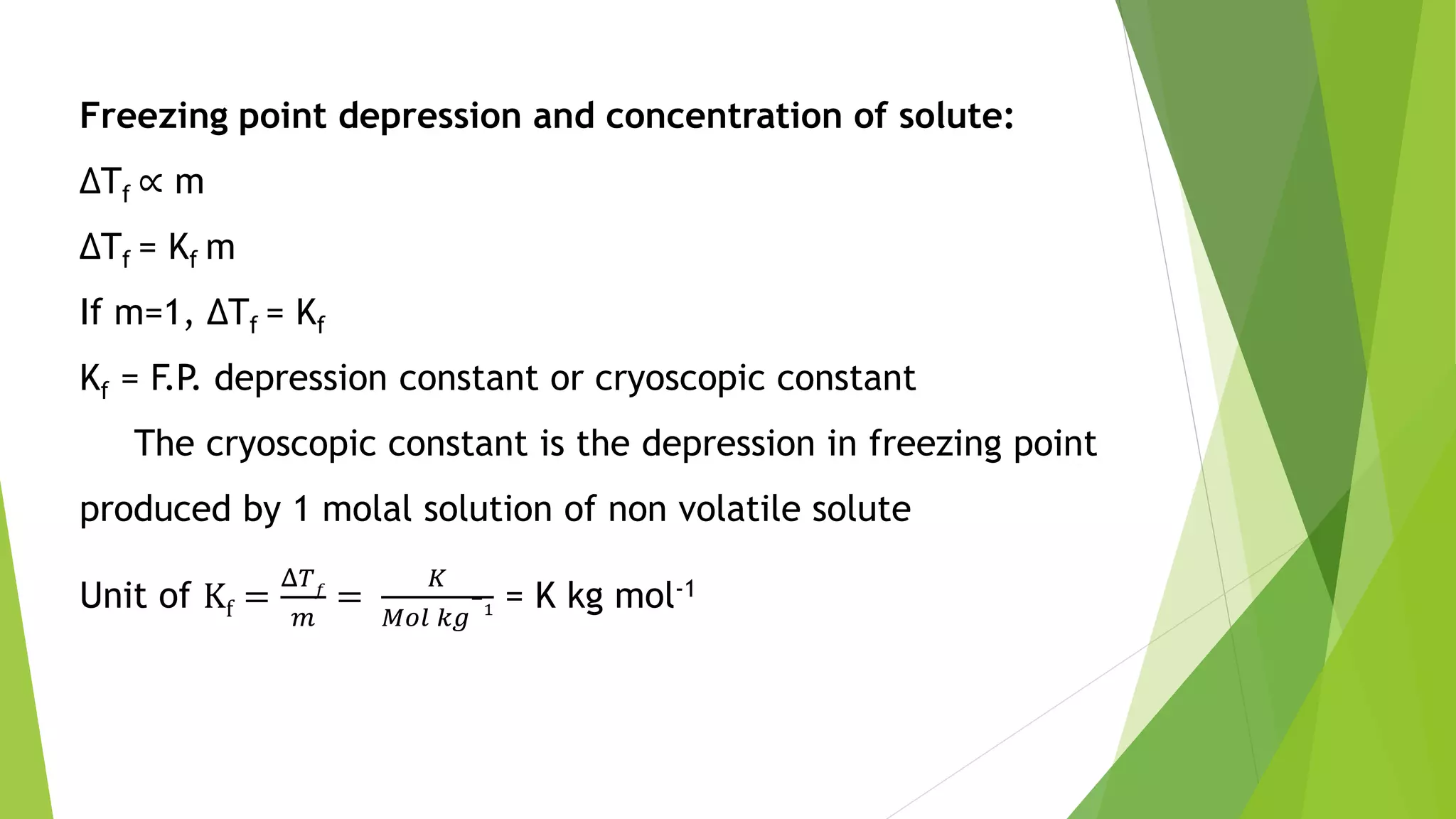
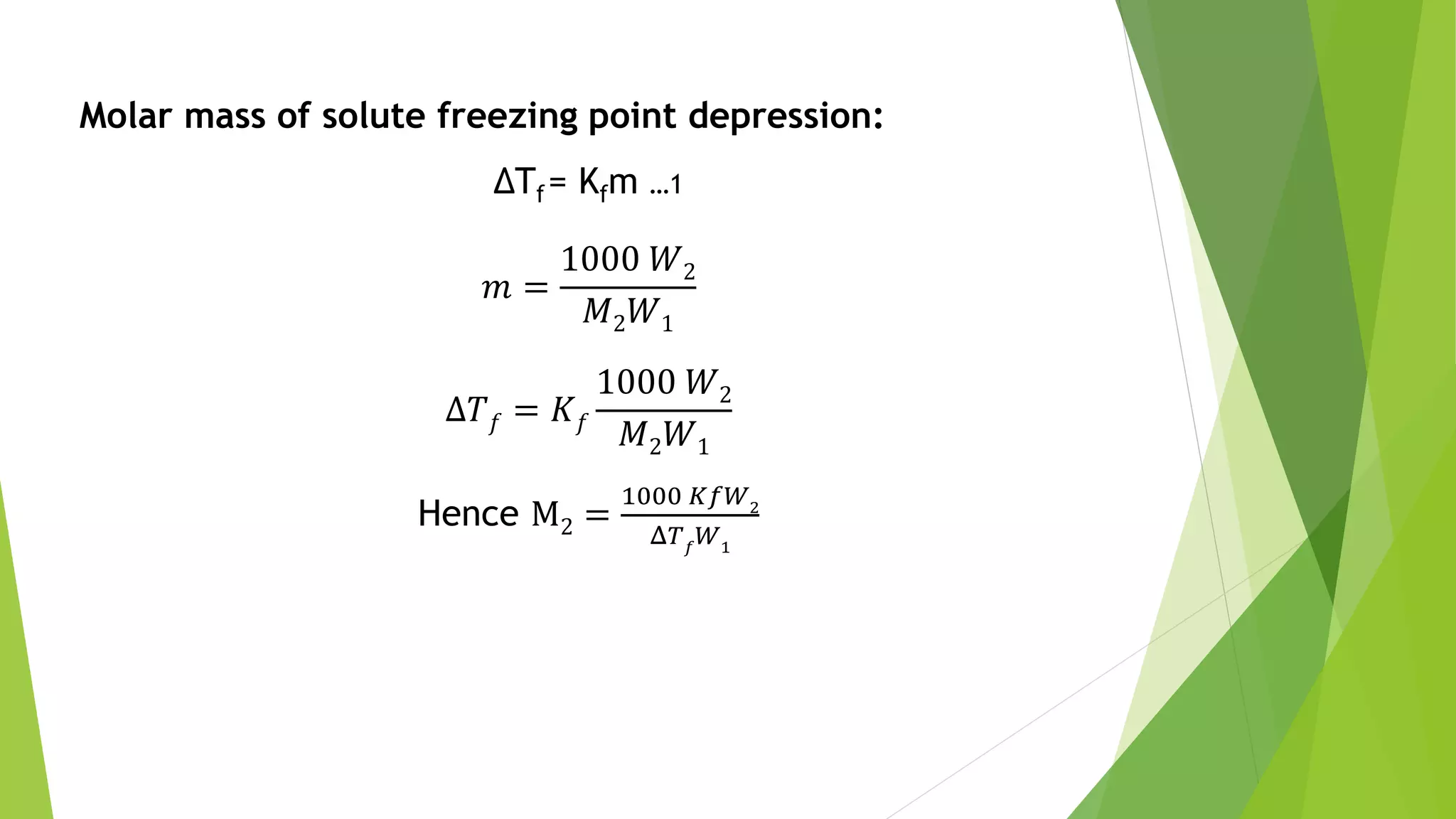
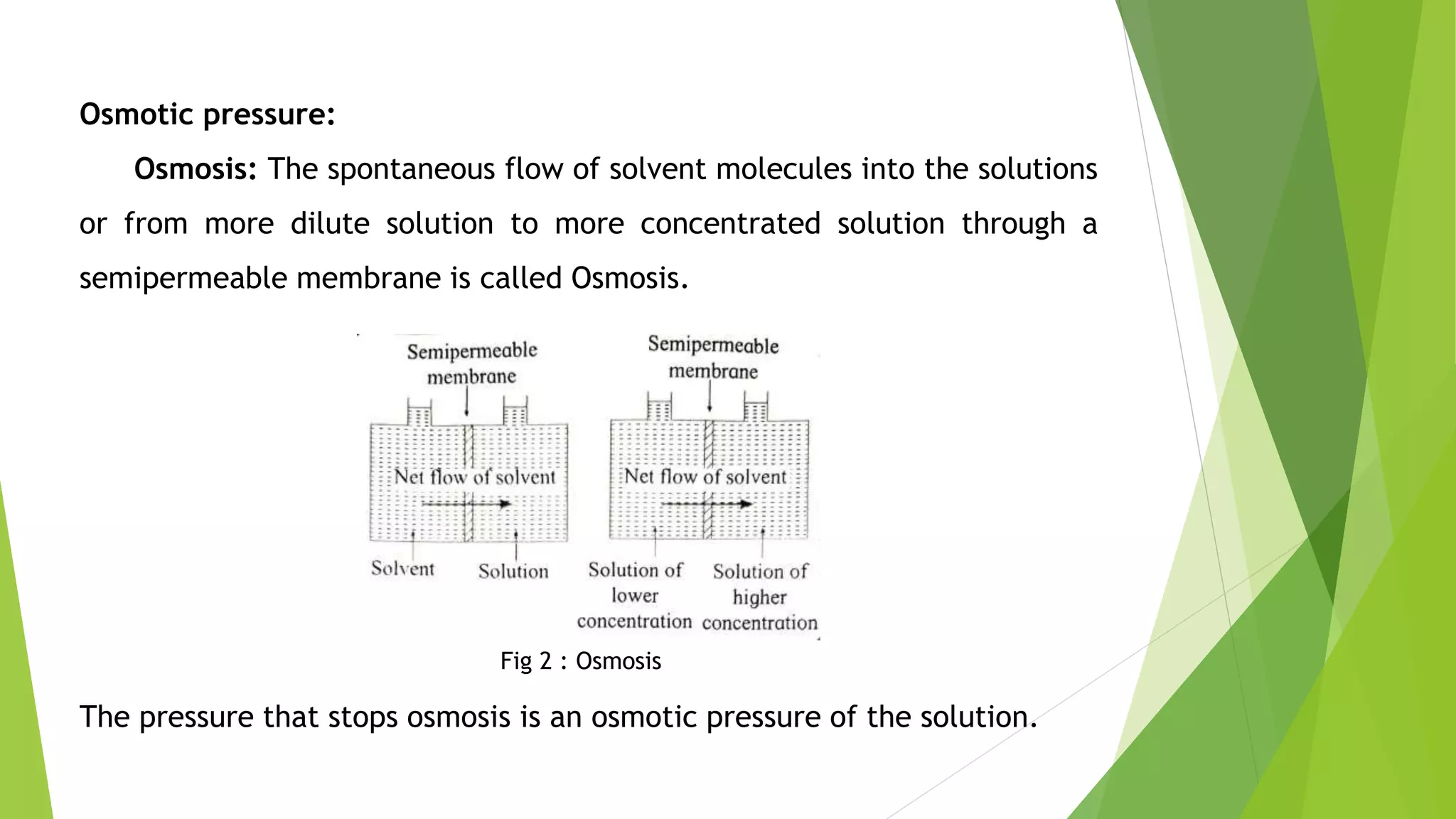
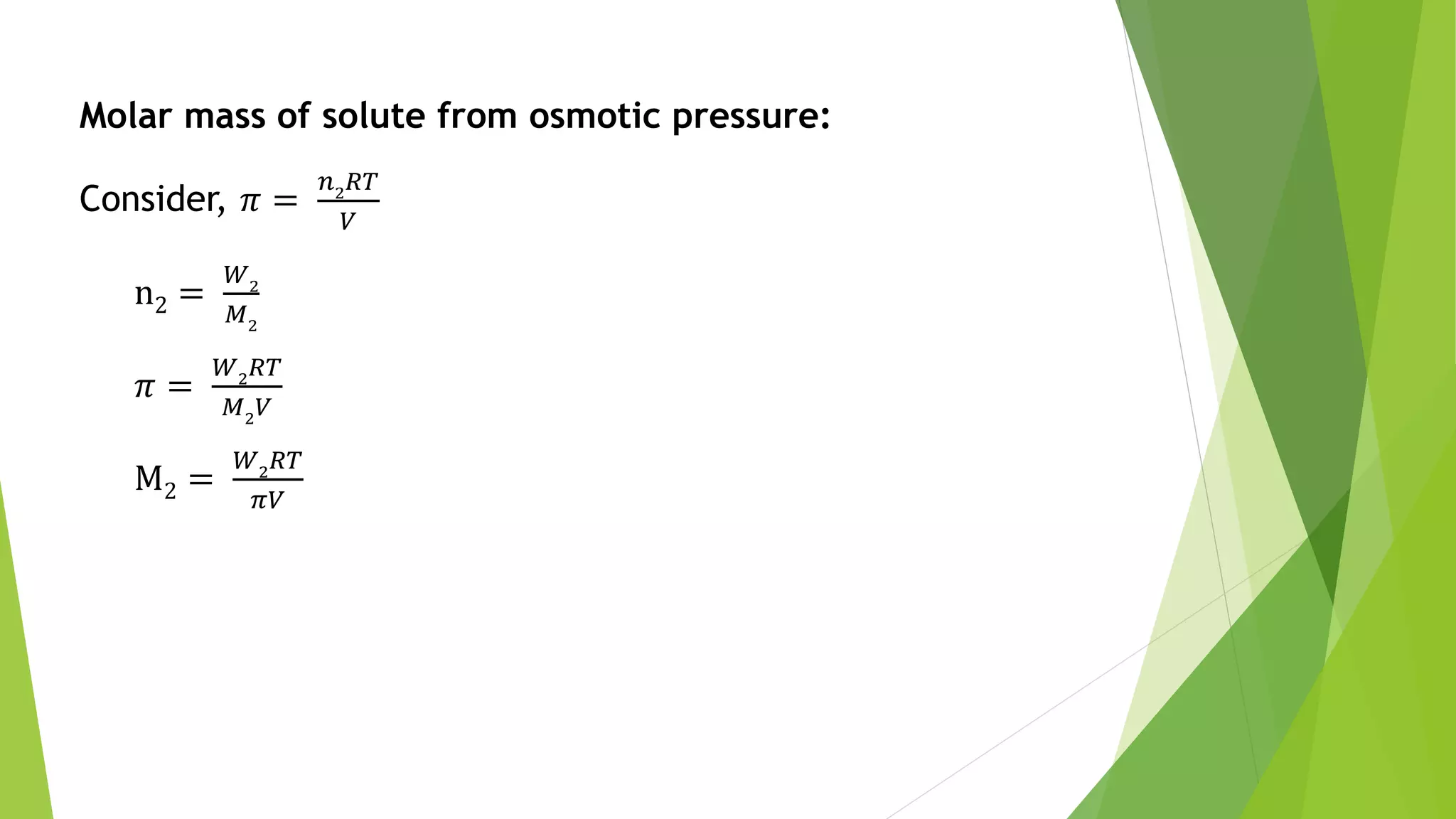
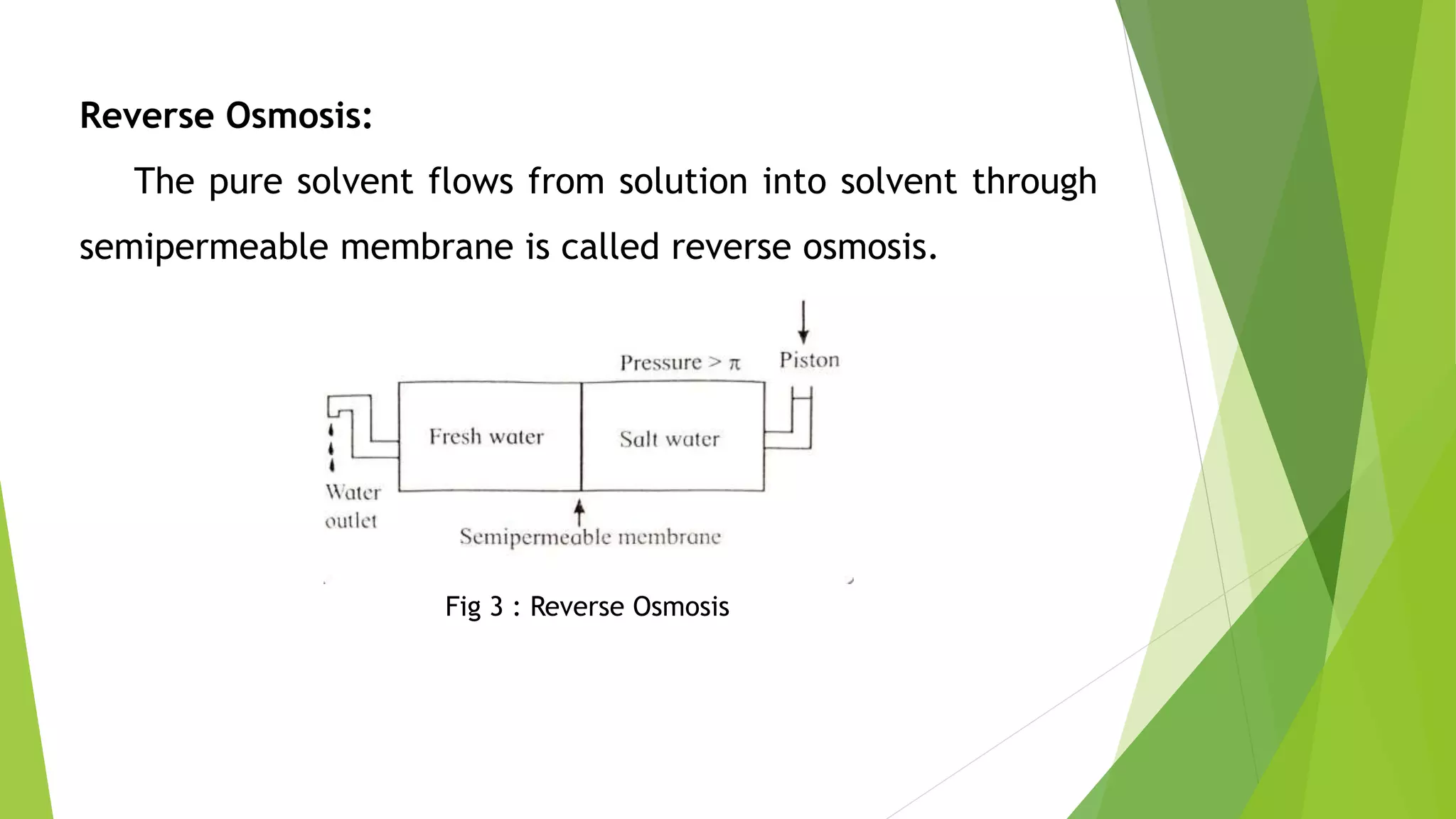
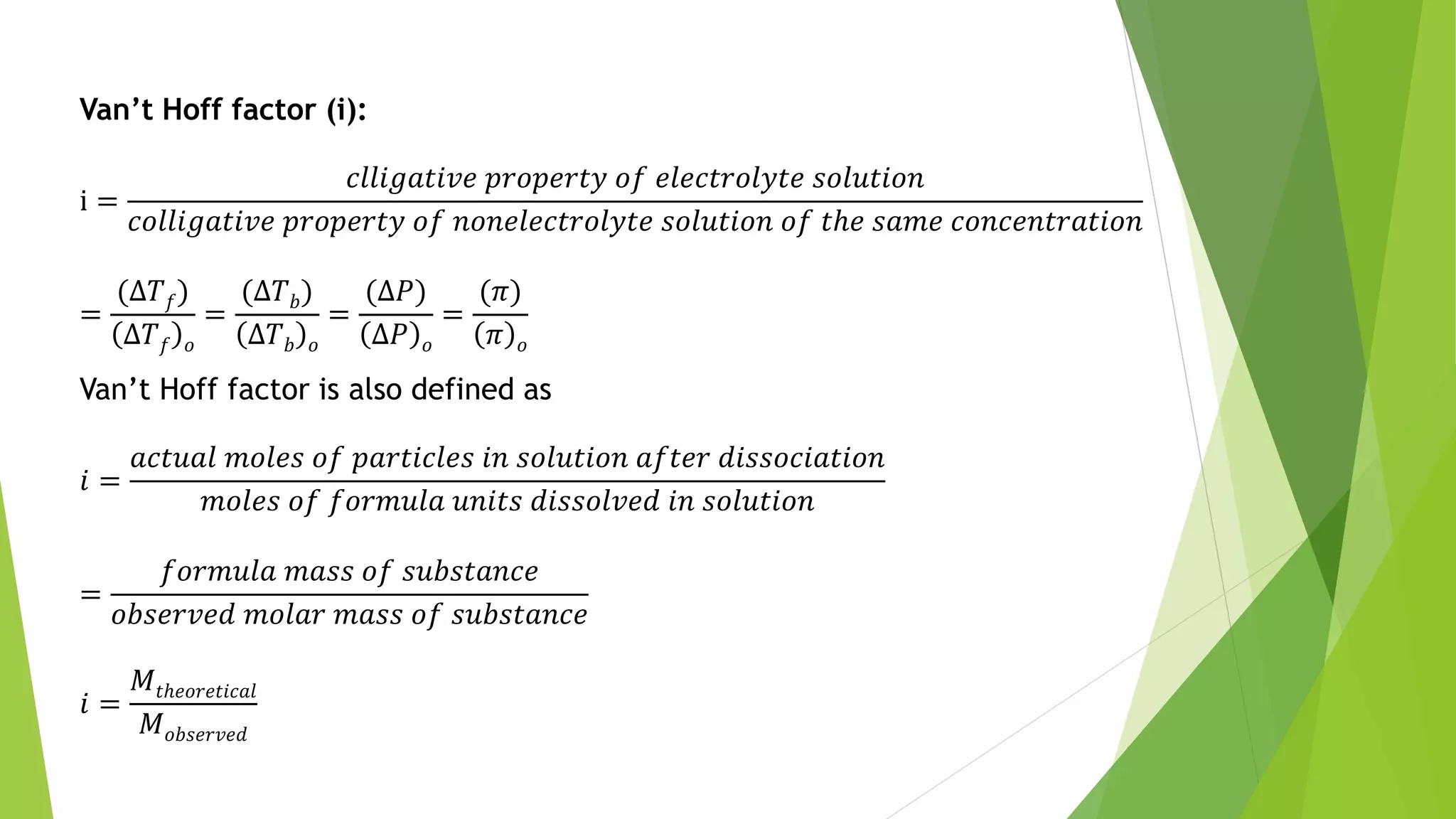
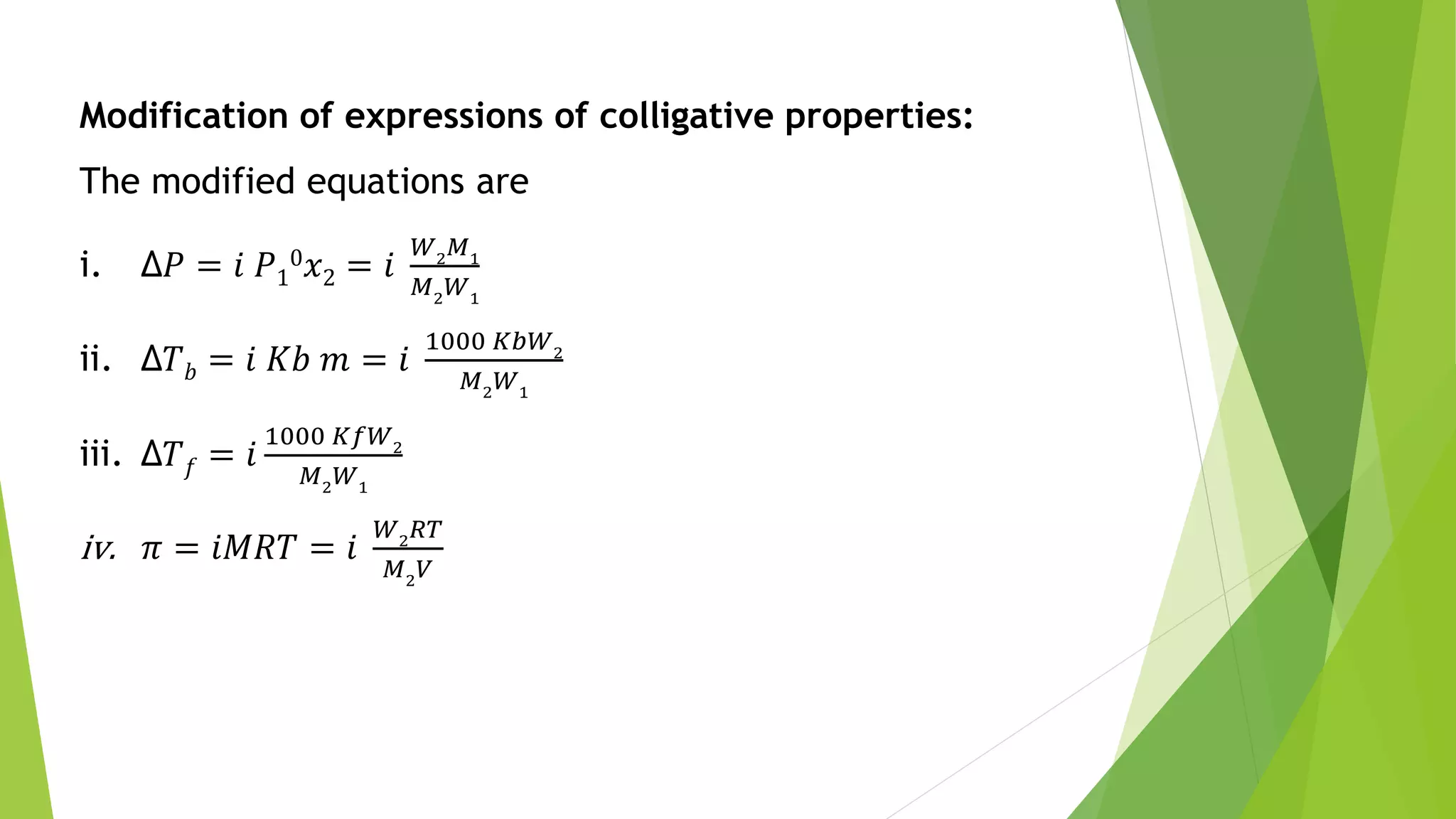
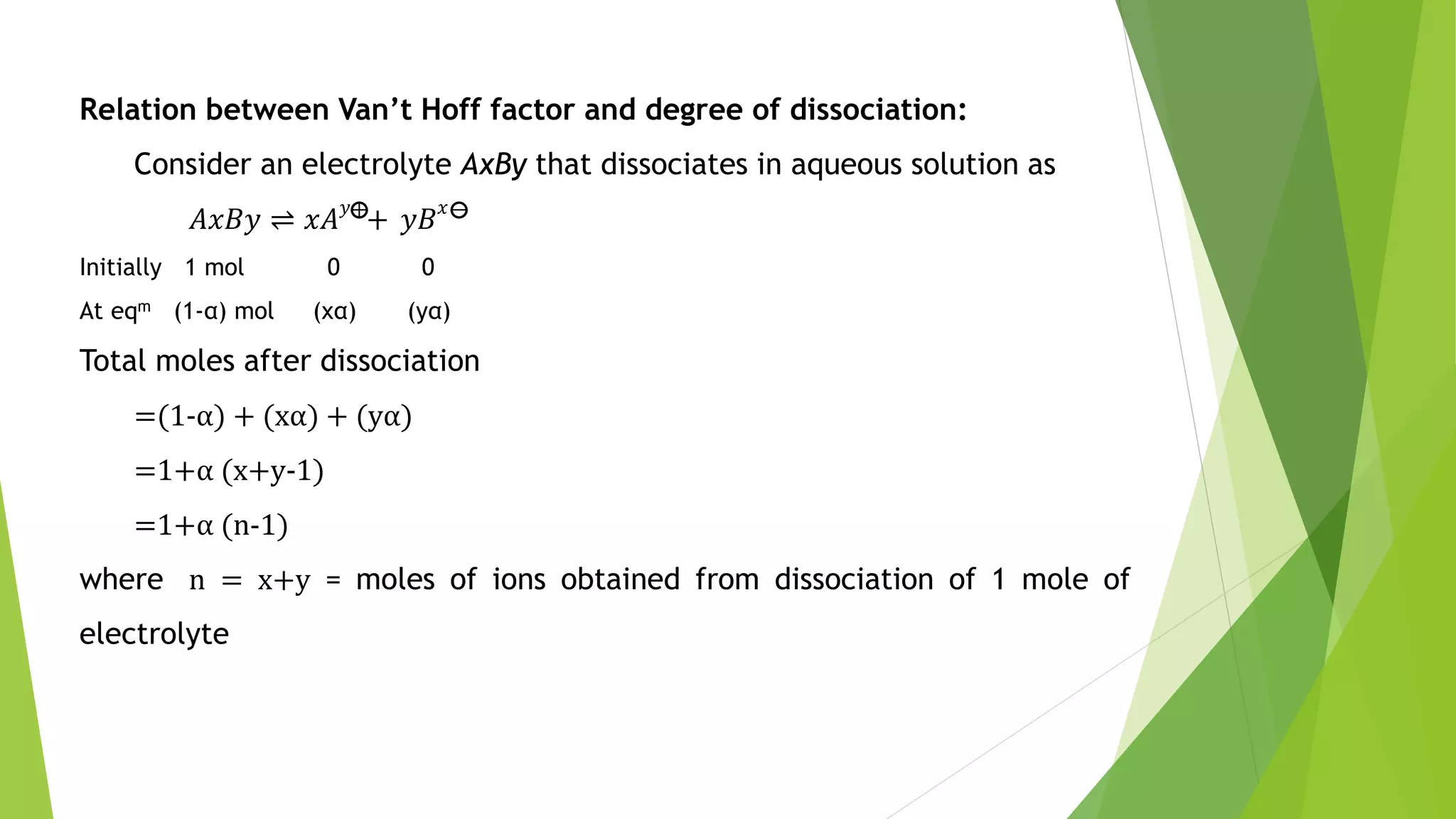
This document discusses colligative properties of solutions such as freezing point depression, boiling point elevation, and osmotic pressure. It explains that these properties are affected by the number of particles in solution. For electrolyte solutions, the properties are greater than nonelectrolyte solutions of the same concentration due to dissociation of ions. The document also defines terms like isotonic, hypertonic, and hypotonic solutions and introduces the Van't Hoff factor to relate colligative properties of electrolytes to their degree of dissociation.