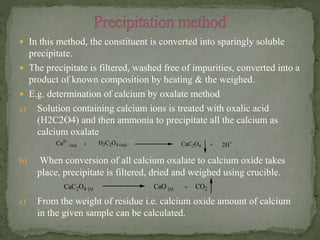
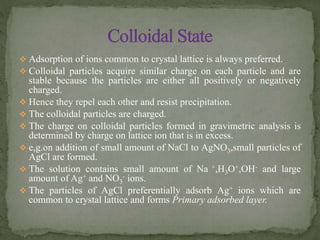
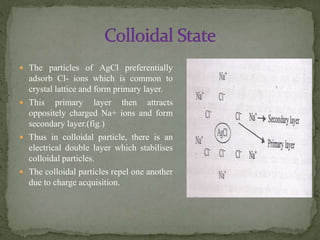
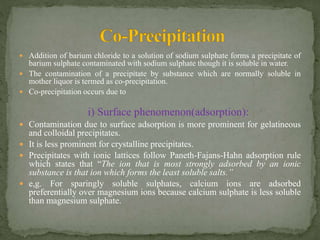
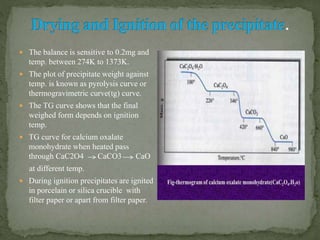
Gravimetric analysis is a quantitative analytical technique where the analyte is converted into a precipitate and weighed. There are two major types - precipitation and volatilization. In precipitation, the analyte forms an insoluble precipitate which is filtered, dried, and weighed. Factors like temperature, pH, and common ion effect influence the completeness and solubility of precipitates. Particle size and purity depend on factors controlling nucleation and growth during precipitation. Gravimetric analysis provides highly accurate quantitative results.







![1. Common ion effect :
The solubility of a salt is less in a solution containing ions in common
with that of the salt in water provided the equilibrium is not disturbed by
the formation of complex ions this is known as common ion effect.
The solubility of precipitate is decreased by the presence of other ions in
the solution common with the precipitate.
E.g. during the precipitation of Ag+ ions from an aqueous solution
containing excess of Cl- ions, even small quantity of Ag+ ions in the
solution can get readily precipitated as Cl-ions are present in excess.
Solubility product, Ksp of AgCl is constant, Ksp = [Ag+] [Cl-]
In some cases excess of precipitant decreases the solubility of precipitate
but in some cases it increases.
In some gravimetric estimation, organic reagents are used as precipitant
which are prepared in non-polar organic solvents. E.g. dimethyl
glyoximate (DMG) for precipitation of nickel present in solution.](https://image.slidesharecdn.com/analyticalchemistry-gravimetricanalysis-171230133112/85/Analytical-chemistry-gravimetric-analysis-10-320.jpg)
![2. Diverse Ion Effect or Salt Effect
Solubility of a sparingly soluble salt increases in presence of
foreign ion, that is ions which are not common to those of the salt.
This effect is called diverse ion effect, salt effect or activity effect.
E.g. Solubility of BaSO4 is increased by 70% in 0.01M solution of
potassium nitrate than in water.
Potassium nitrate is strong electrolyte and highly soluble in water
and its solution contains a high conc. of ions. This results increase
of ionic strength of the solution.
The activity ‘a’ is related to the molar concentration ‘c’ by the
activity coefficient ‘γ’ as
a = c * γ
e.g. solubility product Ksp for AgCl is written as
Ksp = a Ag+ * a Cl- = [cAg+ * γ Ag+] x [cCl- * γ Cl-]
II. Factor affecting Solubility of precipitate](https://image.slidesharecdn.com/analyticalchemistry-gravimetricanalysis-171230133112/85/Analytical-chemistry-gravimetric-analysis-11-320.jpg)
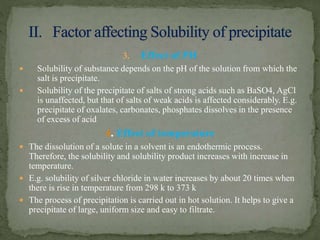







![ Greater the metastable
region[A-C],larger is the
particle size as in BaSO4.
Smaller the metastable
region[A-B],smaller is the
particle size as in AgCl.
When the metastable region is
very small[D-E],a gelatinous or
flocculent precipitate forms as
in ferric hydroxide.
Thus factor affecting solubility
also affects particle size.](https://image.slidesharecdn.com/analyticalchemistry-gravimetricanalysis-171230133112/85/Analytical-chemistry-gravimetric-analysis-21-320.jpg)












![ The wash solution is mostly employed in hot due to greater solubility of
foreign substance and increased speed of filtration.
Besides choice of suitable wash liquid, the mode of washing is also
important.
The precipitate is washed using a jet of wash liquid thoroughly on the filter
paper.
Subsequently the edges of filter paper should be washed.
A large number of small washing using small volume of wash solution is
better and more efficient to remove impurities than a small number of large
washings using large volume of wash solution.
A mathematical expression can be used as
Xn = Xo [ u/u + v] n
Where Xn = Concentration of impurity before washings.
Xo = Concentration of impurity after n washings.
u = cm3 of wash solution retained by the precipitate.
v = cm3 of wash liquid used for each washings.
n = Number of washings.](https://image.slidesharecdn.com/analyticalchemistry-gravimetricanalysis-171230133112/85/Analytical-chemistry-gravimetric-analysis-37-320.jpg)



![Advantages of organic precipitants over inorganic precipitants:
1. The precipitates formed are called organometallic compounds have high
molecular weight. Thus a small amount of metal ions yield a large amount
of precipitate. Hence percentage error is reduced.
2. By maintaining proper pH, one metal ion can be precipitated in presence
of other ion without interference.
3. Precipitate can be dried at suitable temp. and weighed. It is less tedious
than ignition and weighing carried out for inorganic precipitation.
4. Organometallic precipitates are less soluble than inorganic precipitates.
Thus accuracy of results is more.
5. The particle size is better than inorganic precipitate. Hence it is better
suited for filtration and washing of precipitates.
Examples.1) Dimethyl Glyoxime[C4H8O2N2]
2) 8-Hydroxy quinoline(Oxine)[C9H7ON]
3)Cupron (a-Benzoin oxime)
4) Salicylaldoxime[C7H7O2N]](https://image.slidesharecdn.com/analyticalchemistry-gravimetricanalysis-171230133112/85/Analytical-chemistry-gravimetric-analysis-42-320.jpg)



