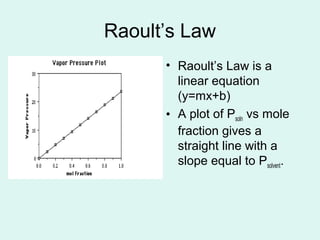
Raoult's law describes the behavior of ideal solutions. It states that the vapor pressure of a solution is proportional to the mole fraction of the solvent in the solution. The vapor pressure of a solution is lower than that of the pure solvent due to the presence of nonvolatile solute particles. This lowering of vapor pressure leads to boiling point elevation and freezing point depression in solutions, as described by the colligative properties. The quantitative relationships for boiling point elevation and freezing point depression involve the molal concentration of the solute.