This document discusses various topics in physical pharmacy and solutions, including:
- Types of solutes such as electrolytes and non-electrolytes.
- Expressions used to quantify concentration in solutions such as molarity, molality, and mole fraction.
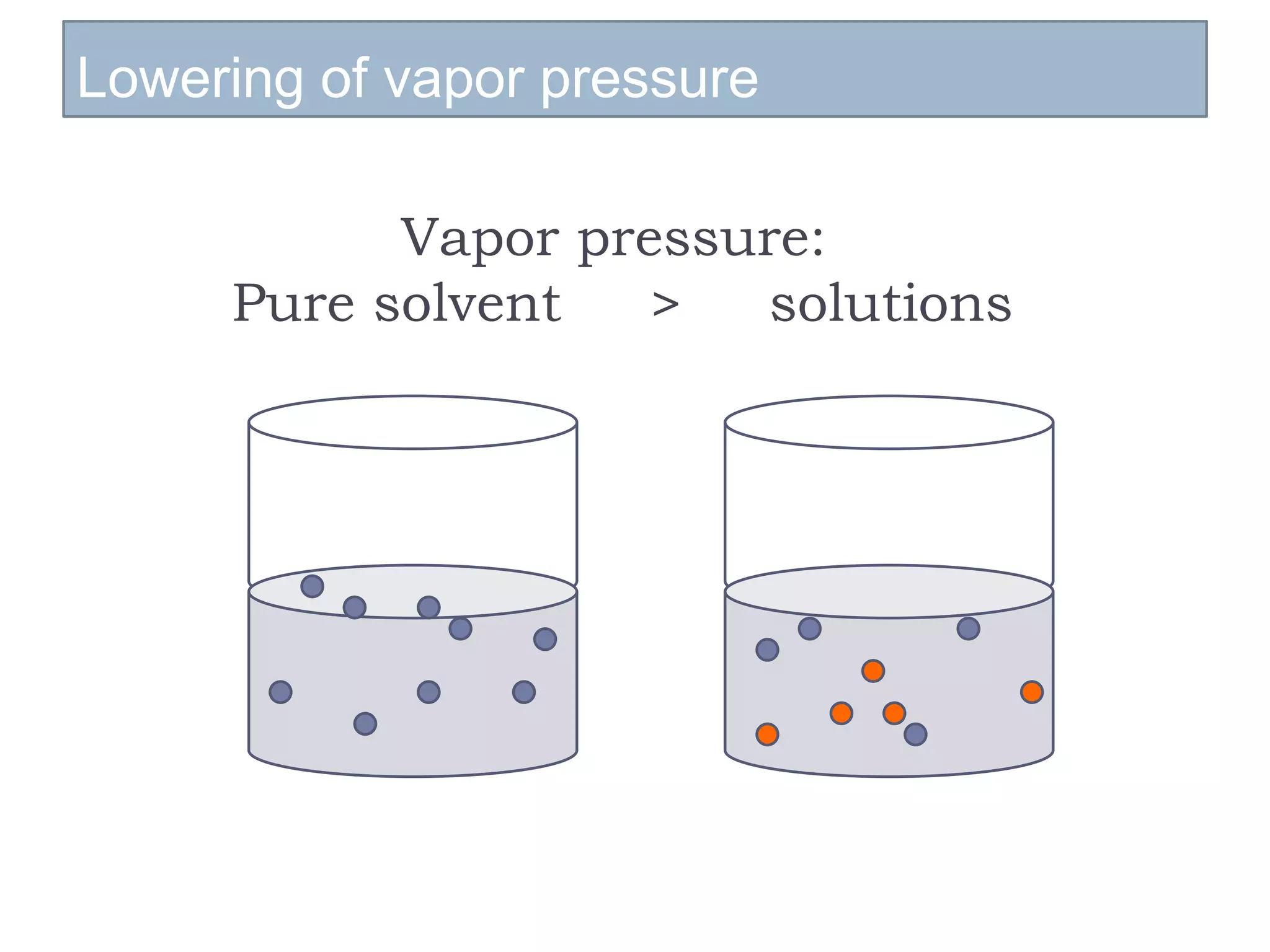
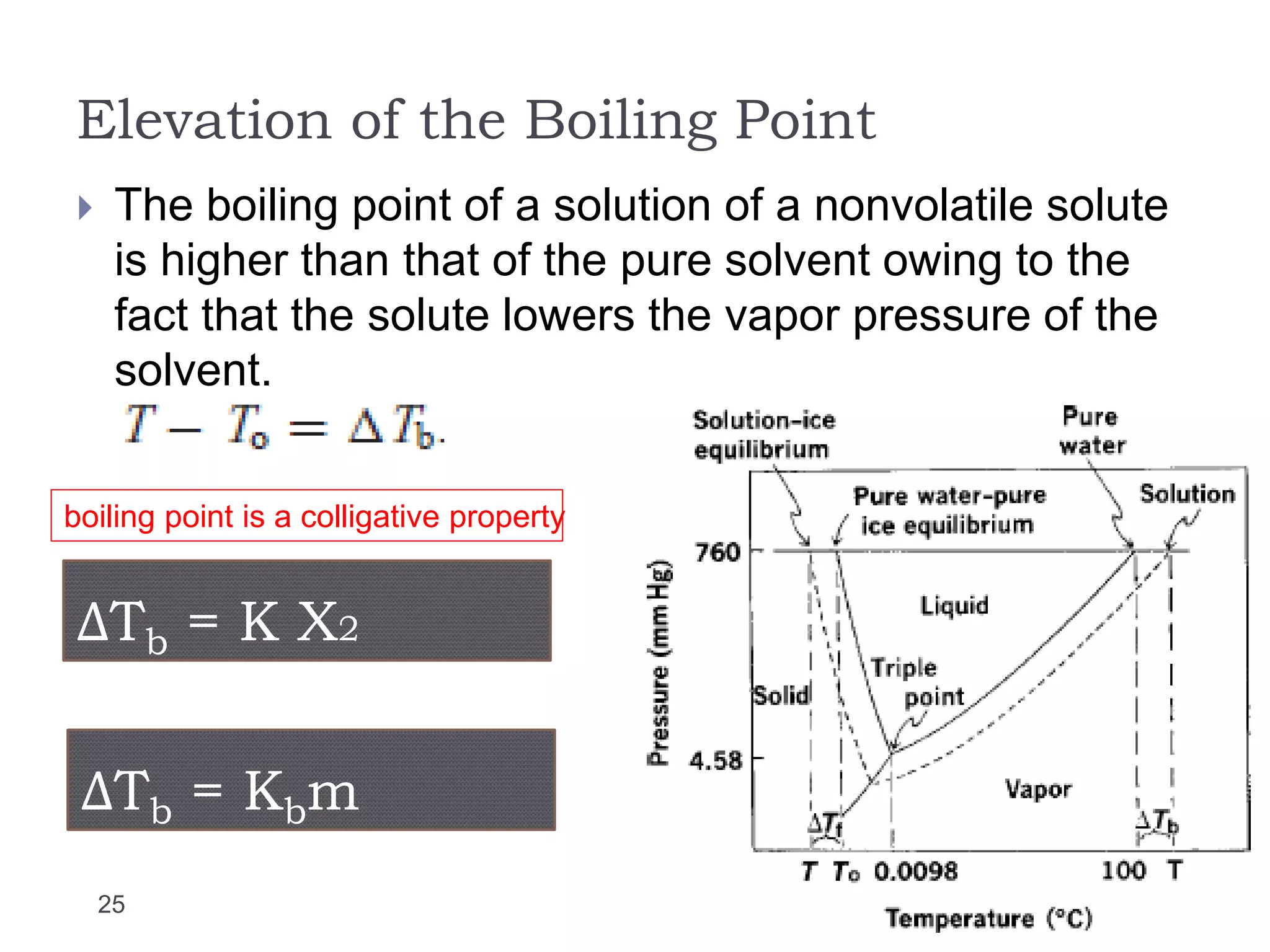
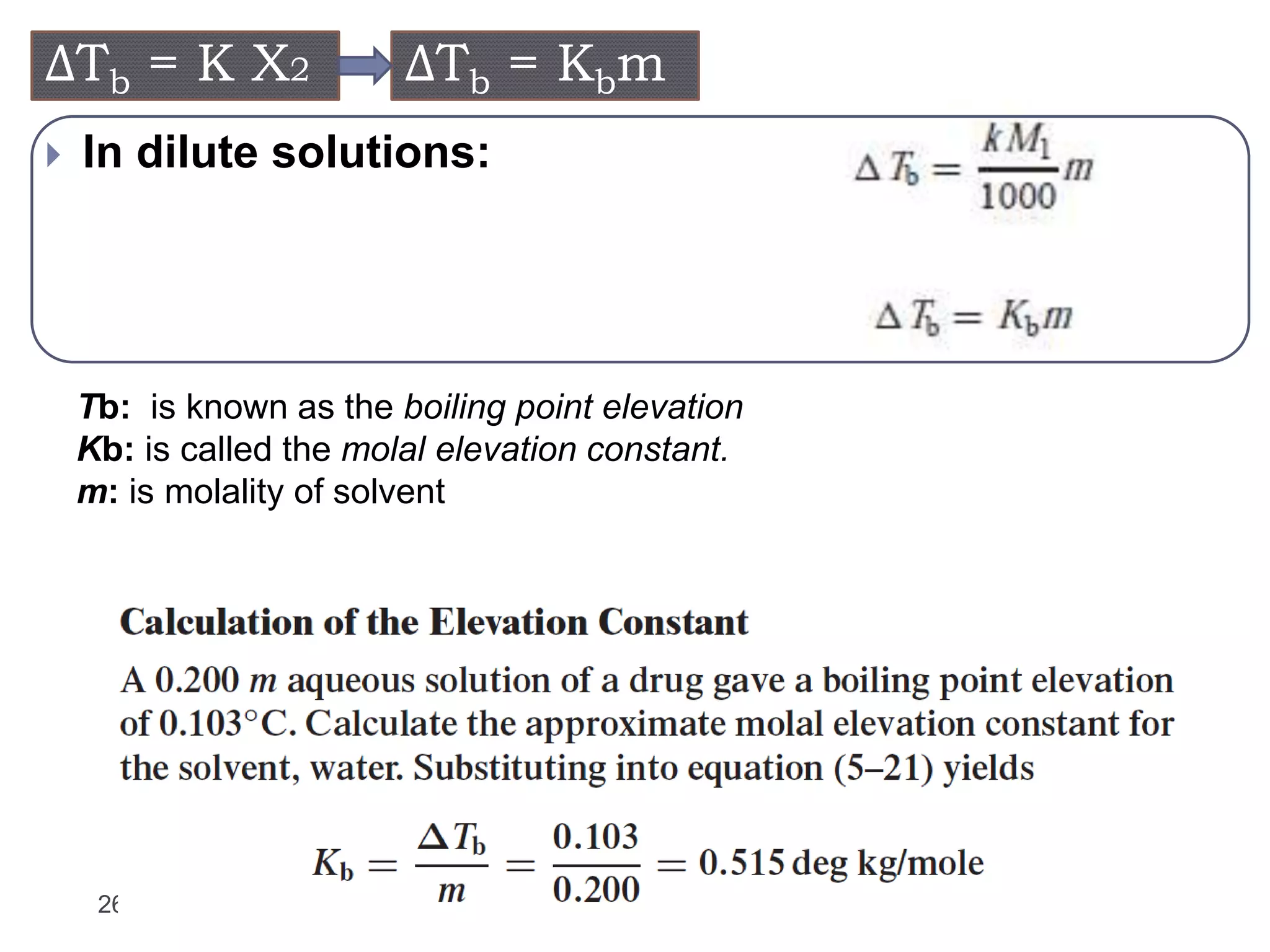
- Factors that influence vapor pressure, boiling point, and freezing point of solutions.
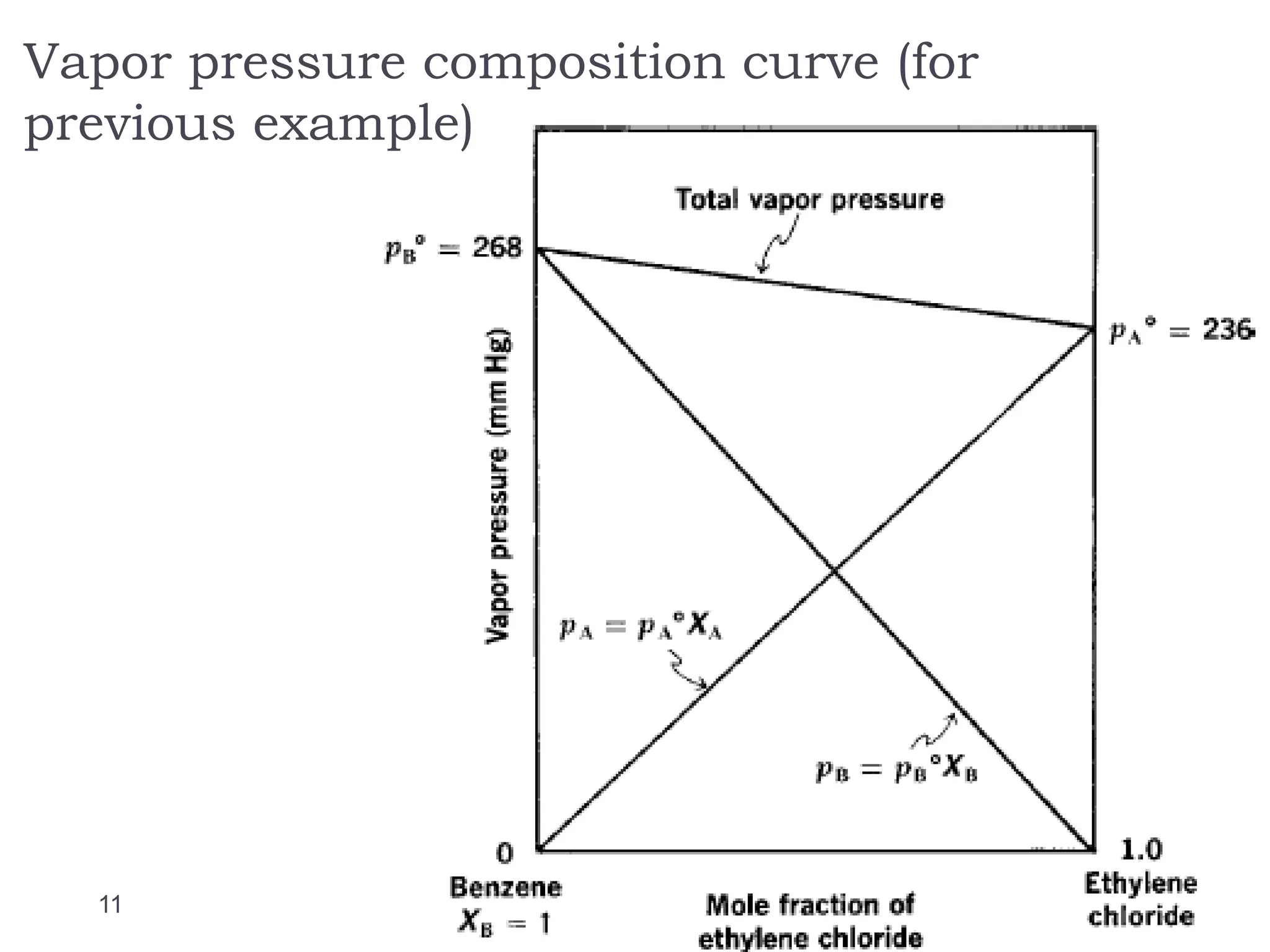
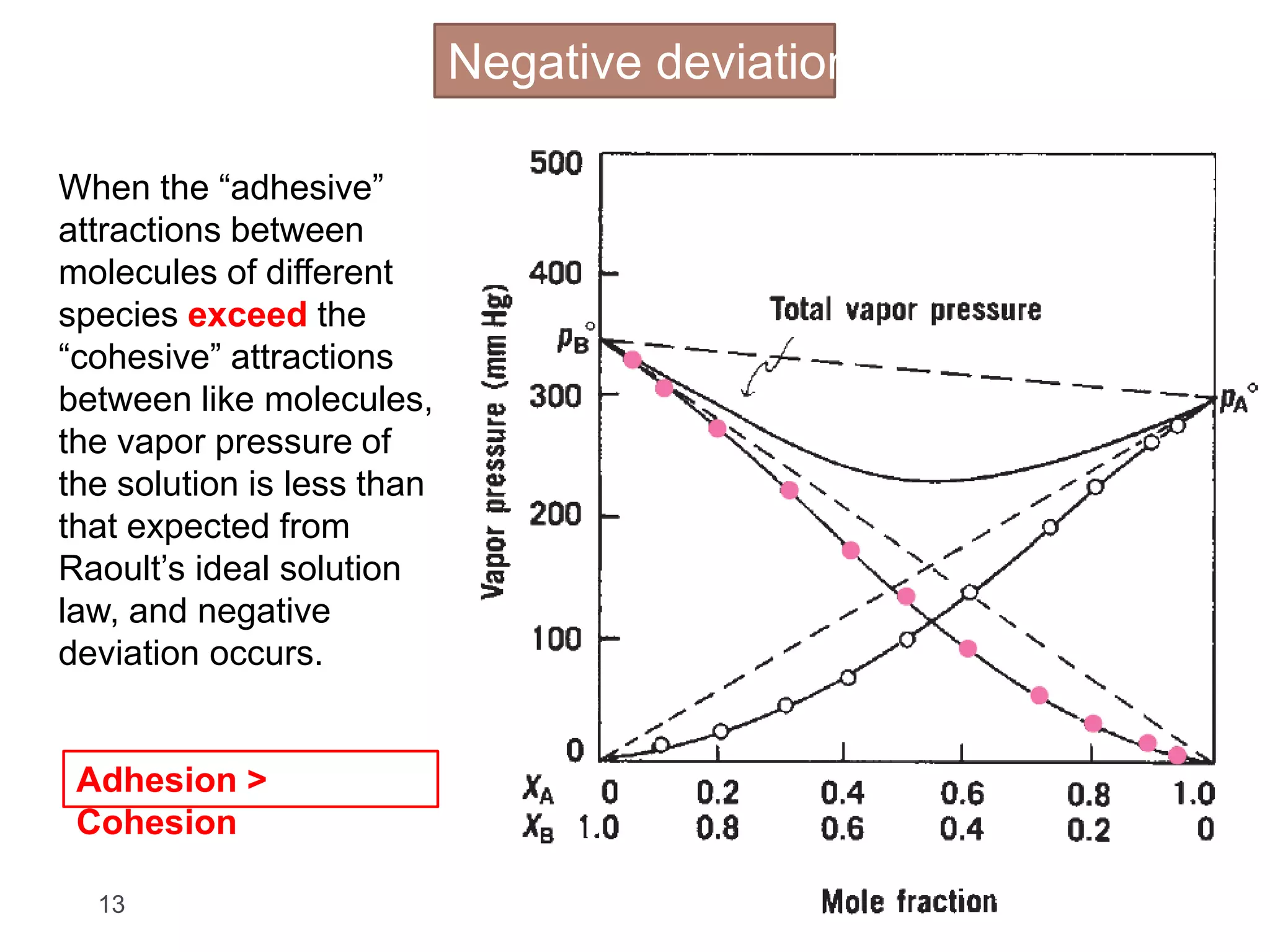
- The concept of ideal and real solutions in relation to Raoult's law and deviations from ideal behavior.
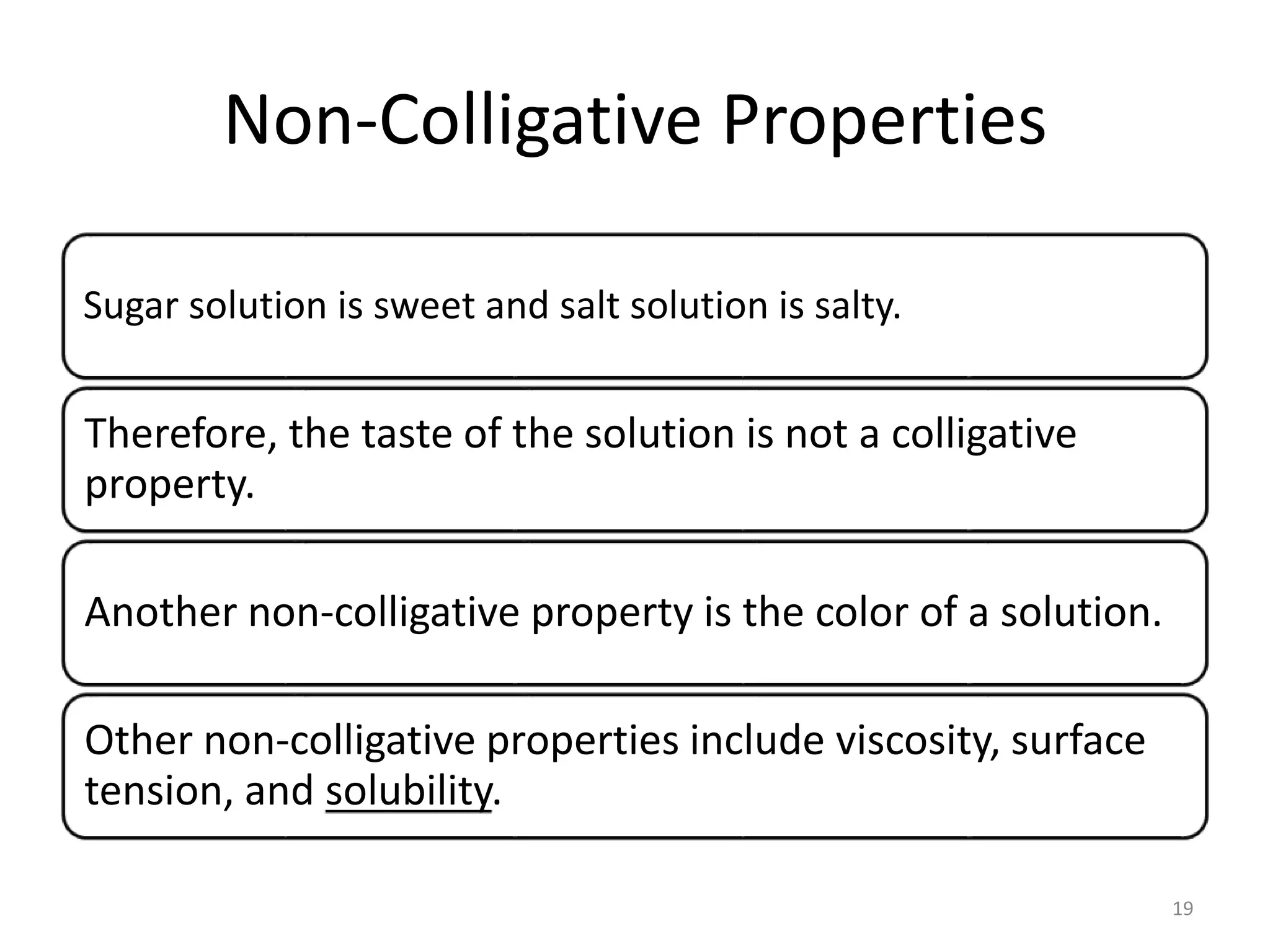
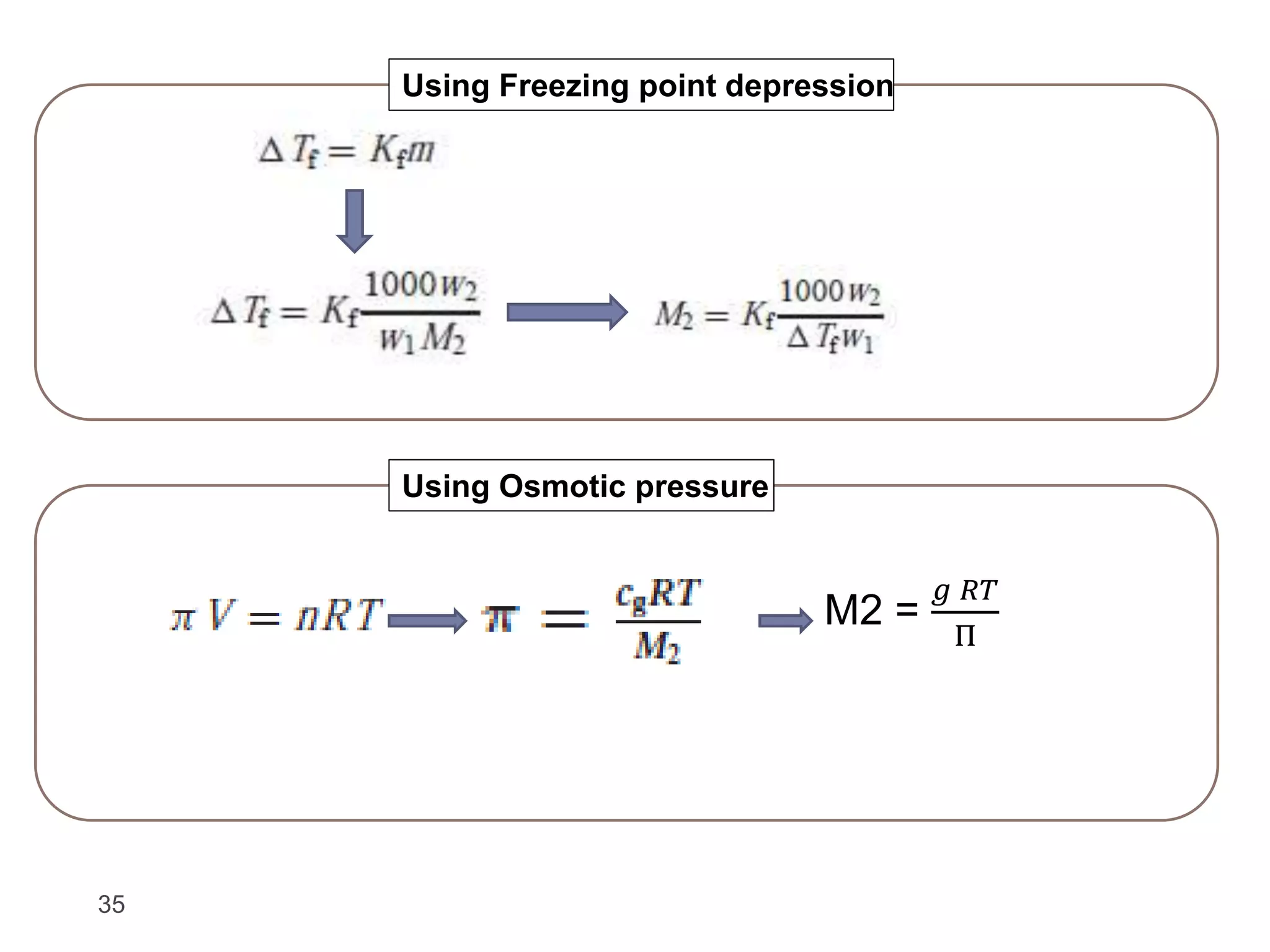
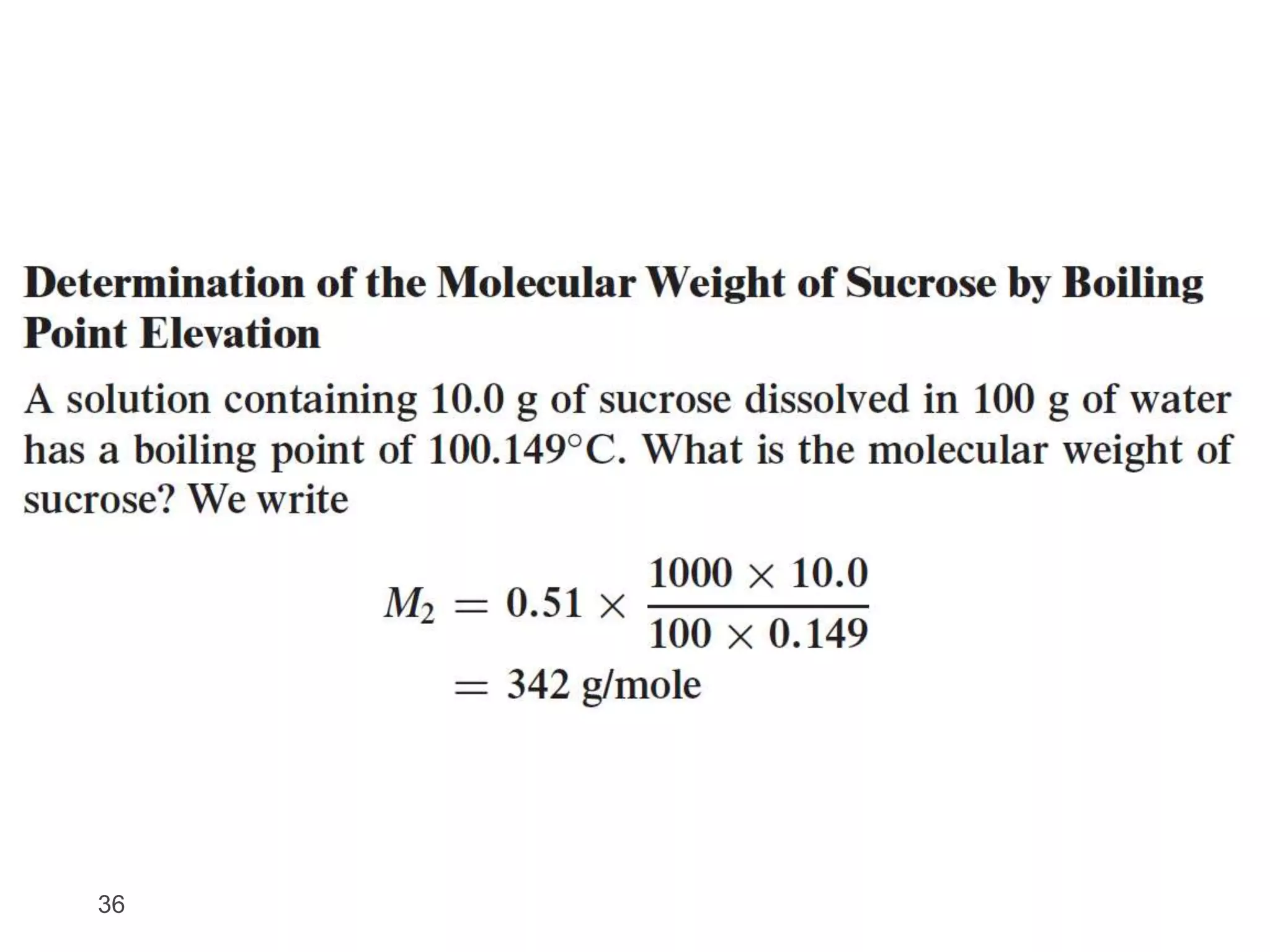
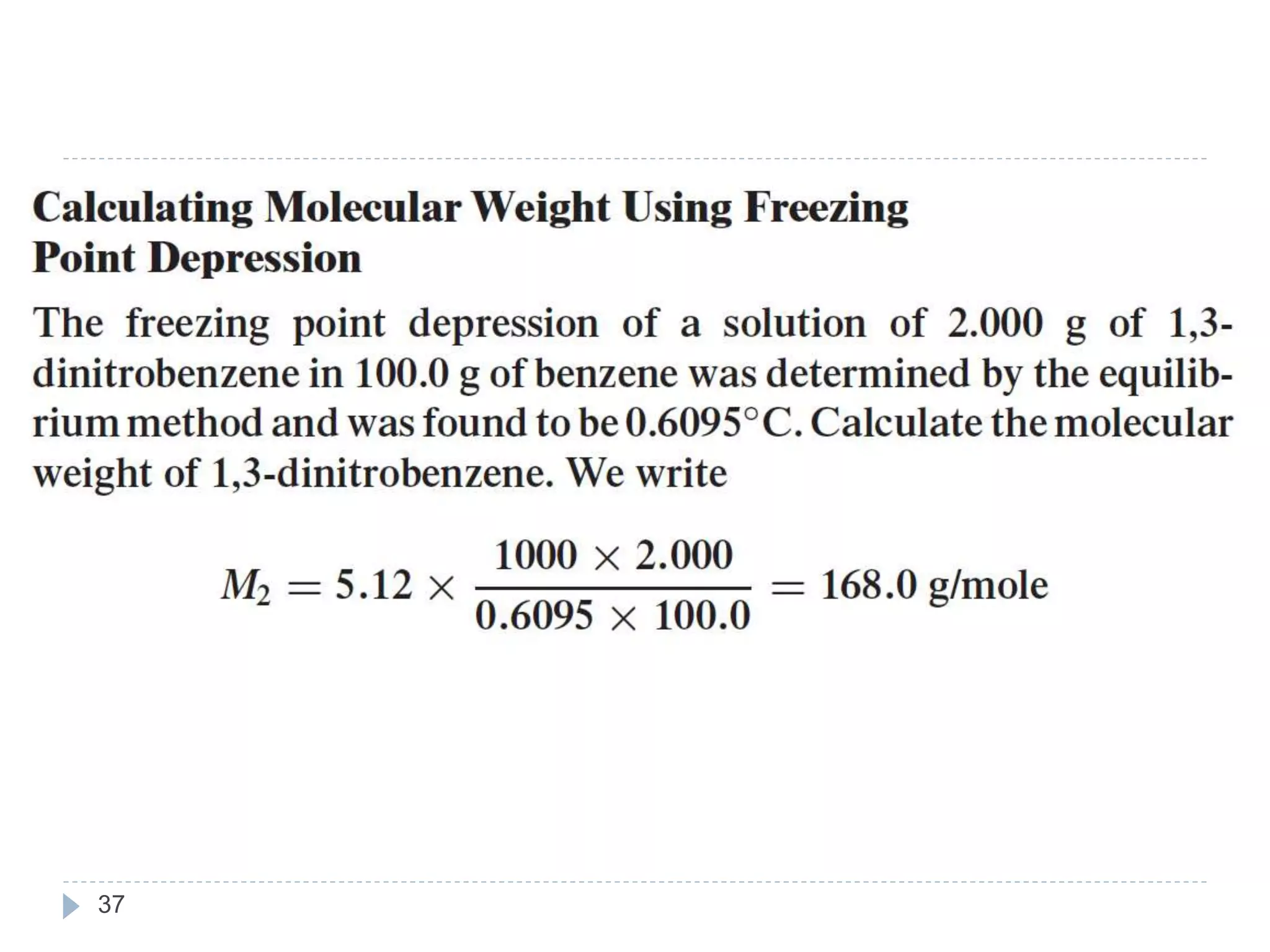
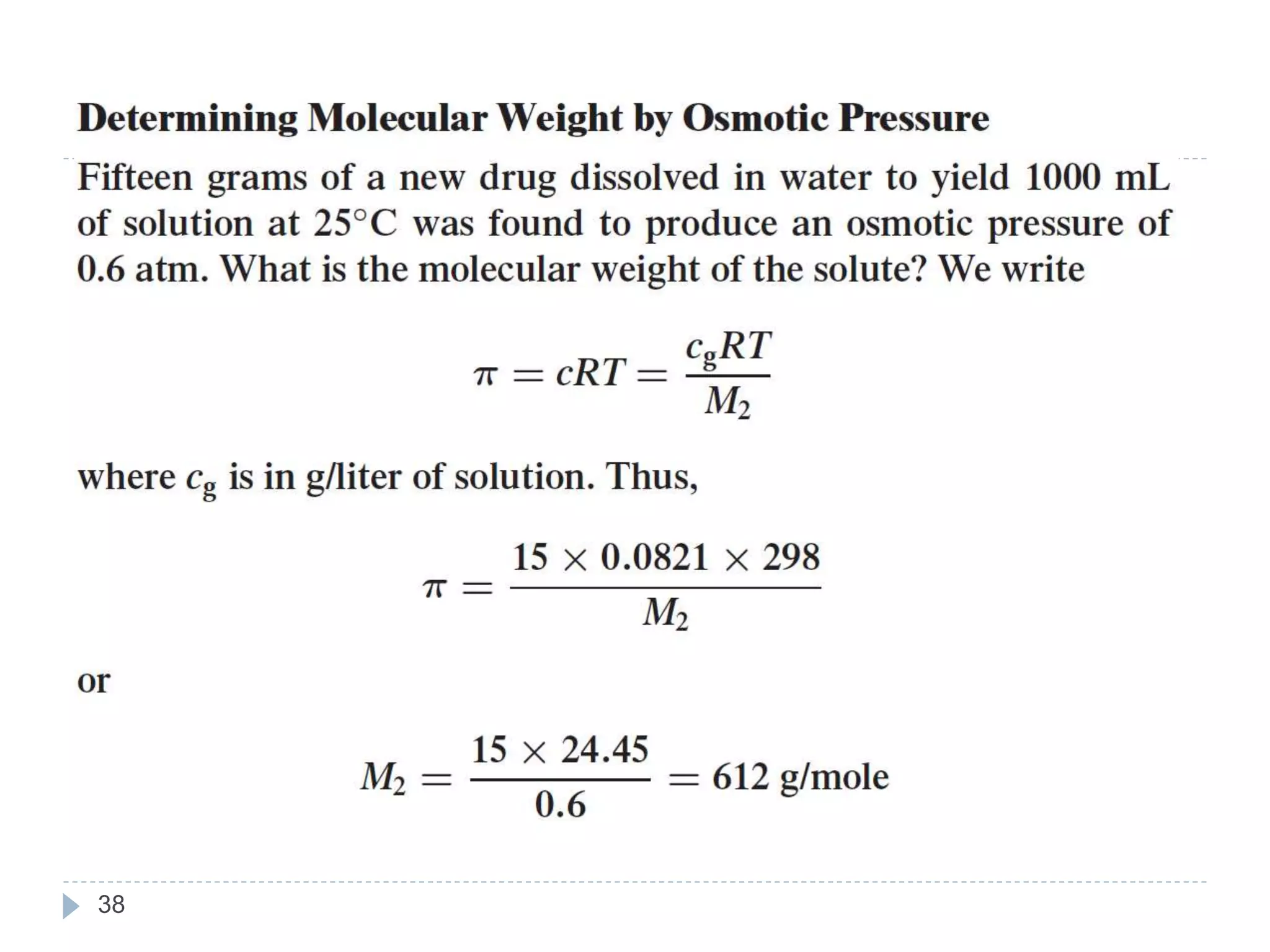
- Colligative properties of solutions that depend only on the number of solute particles, including vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.