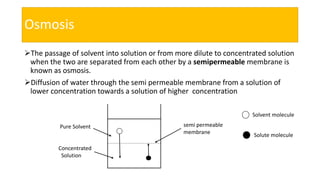
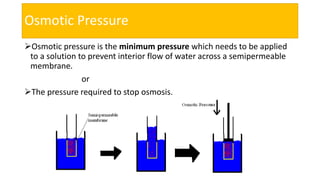
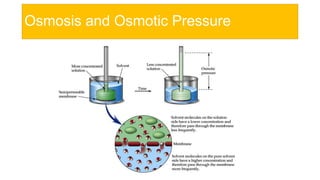
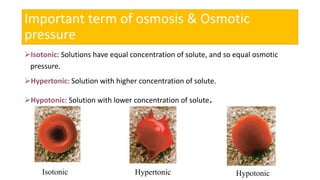
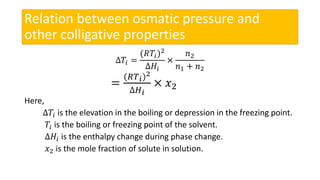
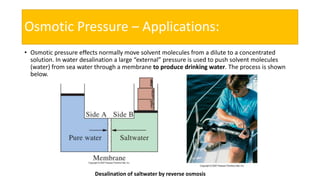
This presentation discusses osmotic pressure and its related concepts. Osmosis is the passage of solvent through a semipermeable membrane from a less concentrated to a more concentrated solution. Osmotic pressure is the minimum pressure required to prevent osmosis, or the flow of water across the membrane. It further defines isotonic, hypertonic, and hypotonic solutions and outlines methods to determine osmotic pressure. Van't Hoff's law relates osmotic pressure to concentration and temperature. Osmotic pressure has important applications including desalination and maintaining fluid balance in the body.