This document summarizes key points about compounds, mixtures, and the differences between physical and chemical properties and changes. It discusses:
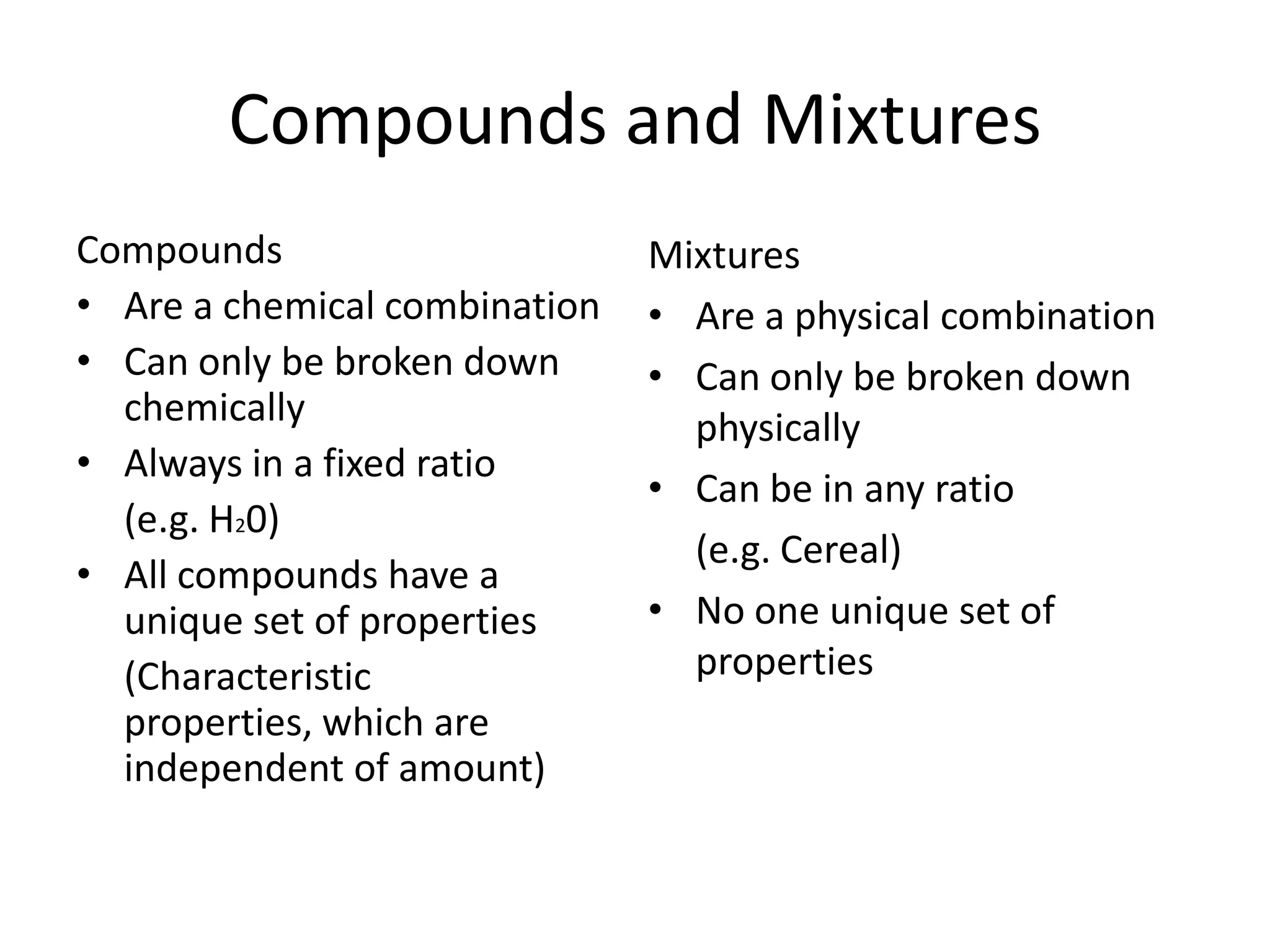
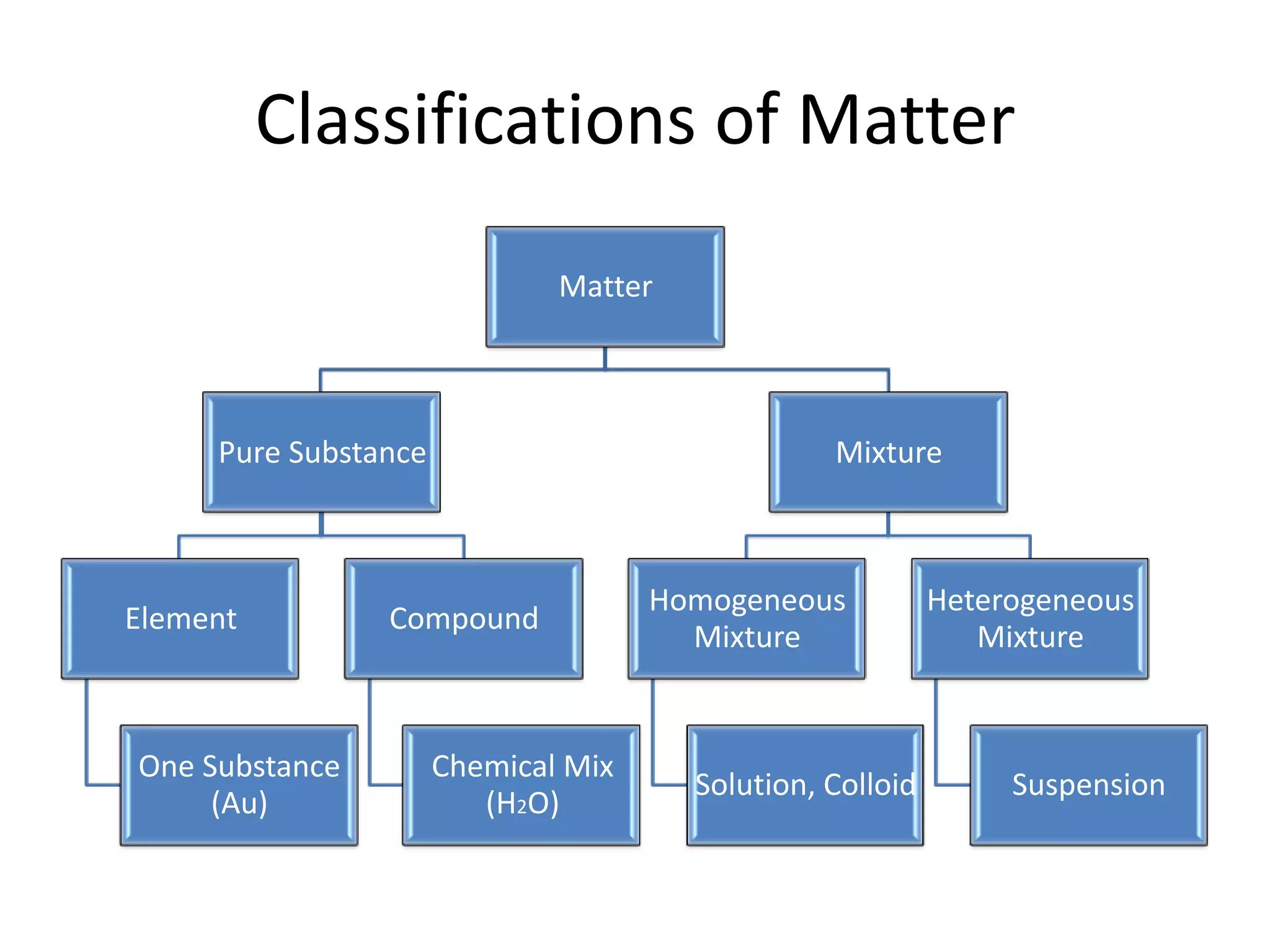
- Compounds are chemical combinations with fixed ratios while mixtures can have varying ratios.
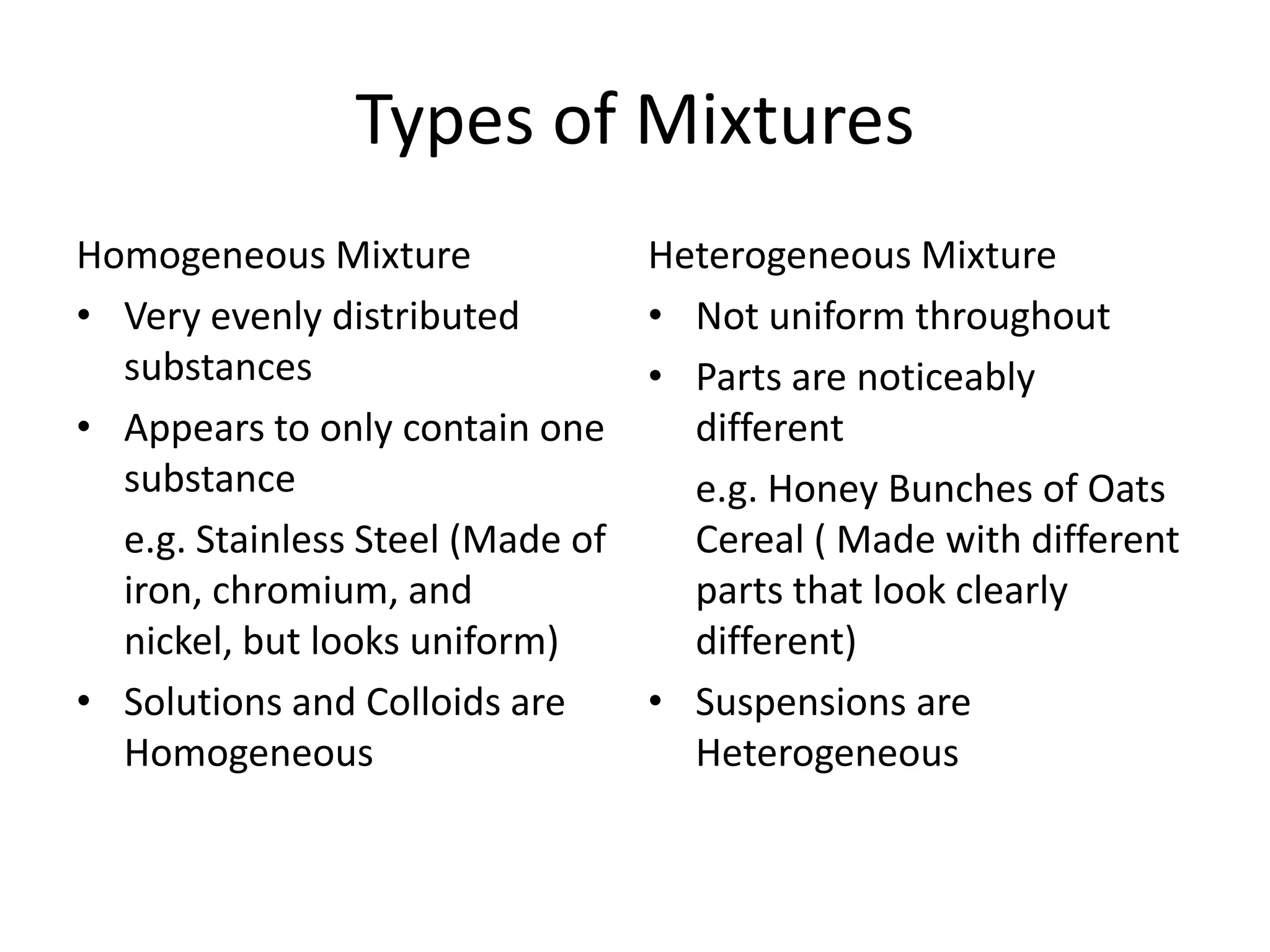
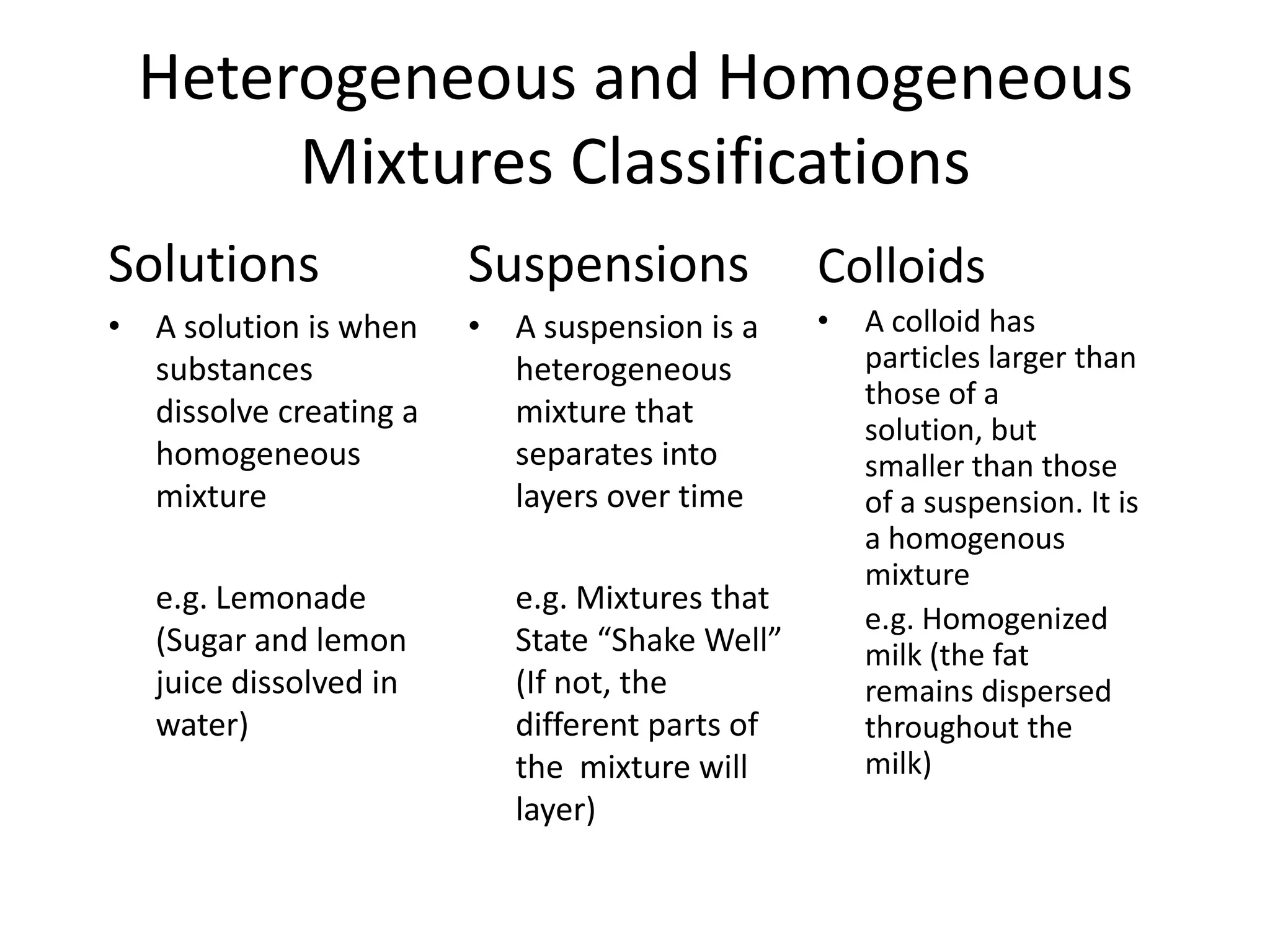
- Mixtures can be homogeneous (uniform) or heterogeneous (non-uniform) and include solutions, colloids, and suspensions.
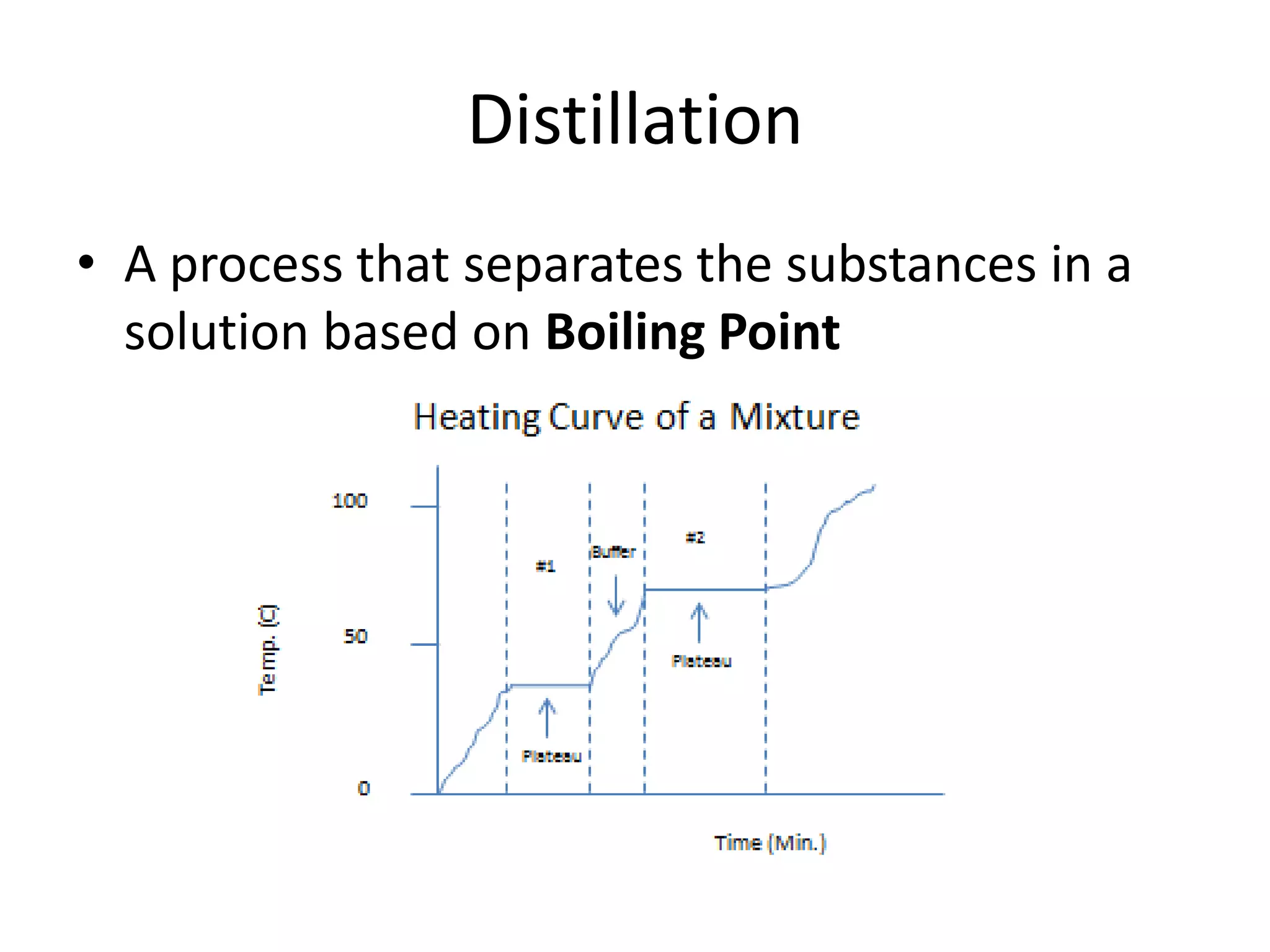
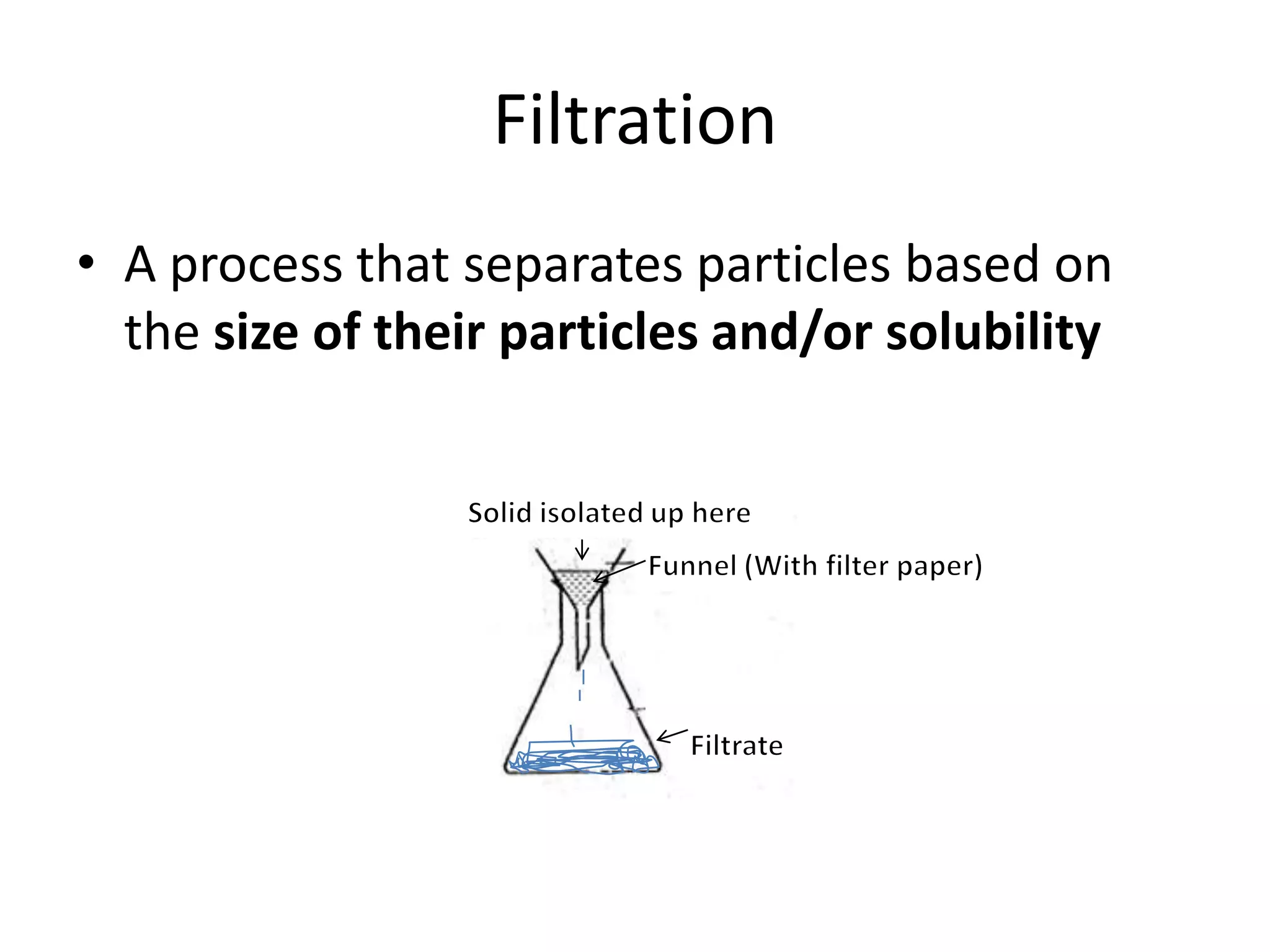
- Physical properties describe observable characteristics without changing a substance's identity, while chemical properties involve substance changes.
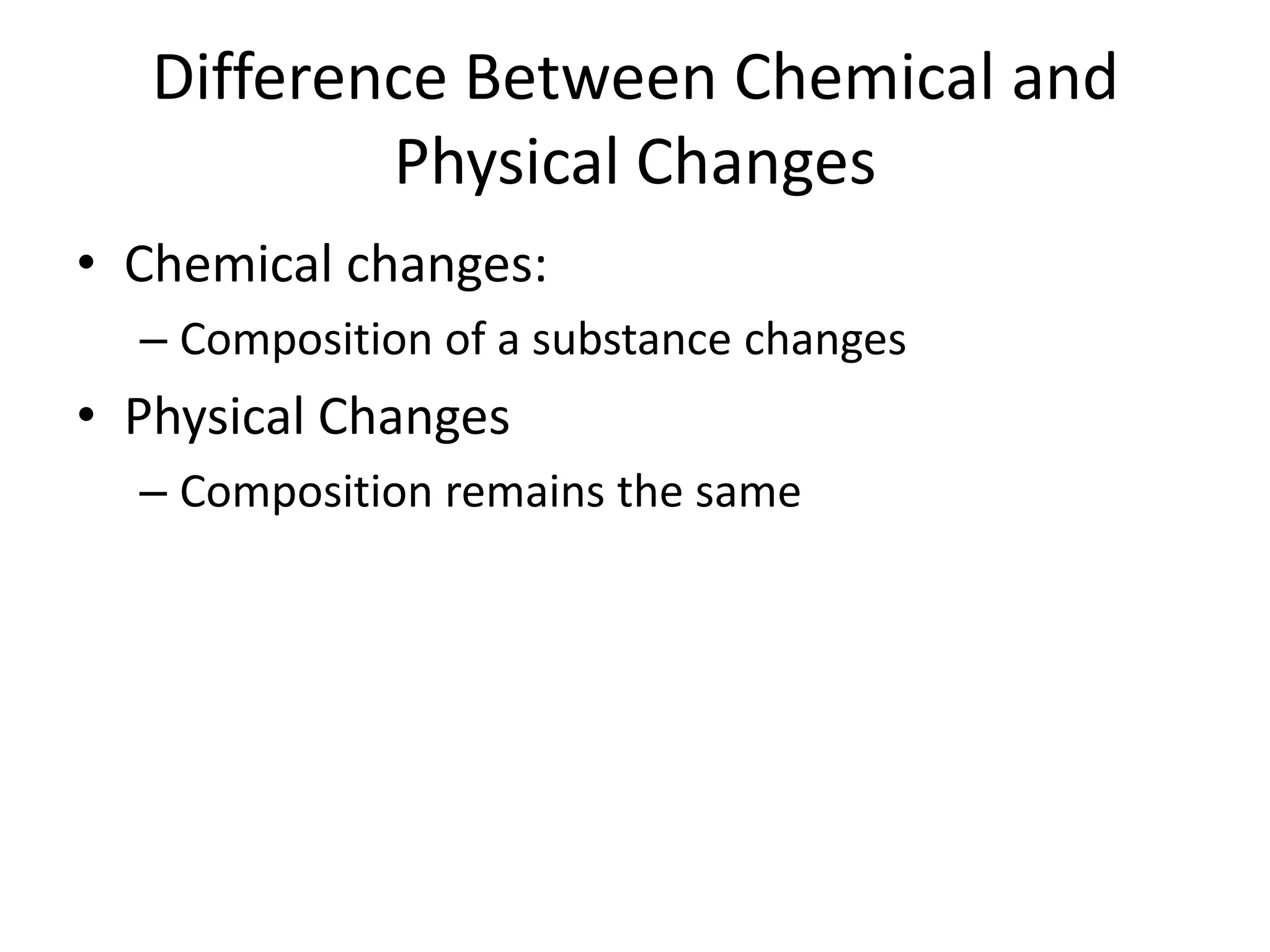
- Physical changes alter appearance/state but not composition, while chemical changes produce new substances with different properties than original reactants.