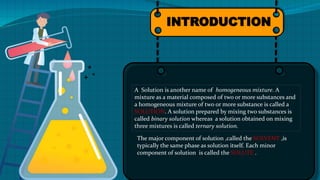
This document provides an introduction to chemistry concepts including solutions, vapor pressure, Raoult's law, and colligative properties. It defines a solution as a homogeneous mixture and discusses solvents and solutes. It then explains vapor pressure and how it relates to Raoult's law for binary solutions. Finally, it discusses the four colligative properties - lowering of vapor pressure, elevation of boiling point, depression of freezing point, and osmotic pressure - and provides examples of Henry's law in daily life like carbonated drinks and decompression sickness in divers.


![VAPOUR PRESSURE
.
The vapour pressure of a liquid is
defined as the pressure exerted by its
vapour phase in equilibrium with liquid
phase at a given temperature. The
vapour pressure is also defined as “the
pressure exerted by vapours of solvent
and solute in equilibrium with the liquid
phase is called vapour pressure of
solution” THE VAPOUR PRESSURE IS
GIVEN AS:
p[solution]=p[solvent]+p[solute]](https://image.slidesharecdn.com/karampreetkaurrollno-221216070006-a610853b/85/Chemistry-7-320.jpg)