Embed presentation
Downloaded 33 times











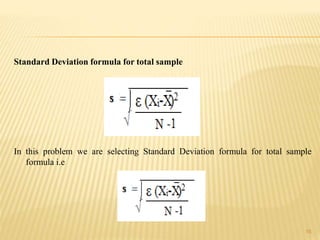

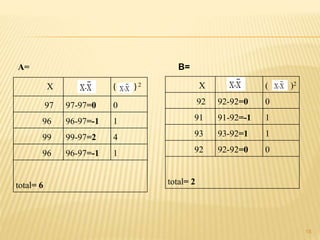
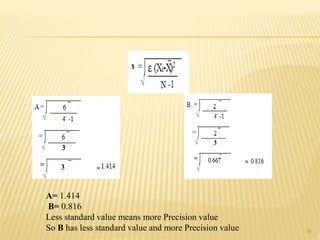
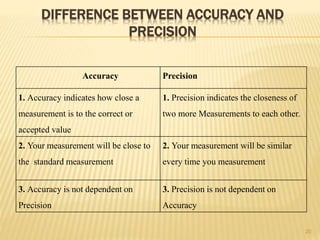
























The document provides an in-depth analysis of errors in analytical chemistry, detailing concepts such as error, accuracy, precision, sources of error, and methods to minimize them. It emphasizes the distinction between determinate and indeterminate errors and discusses various statistical methods, including the Q test, for handling data rejection. Additionally, the document outlines the significance of significant figures and rounding of figures in reporting analytical results.











































