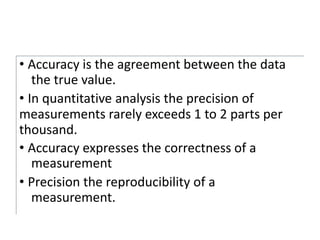
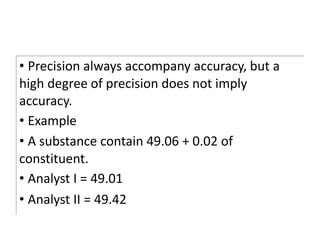
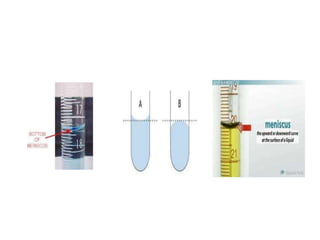
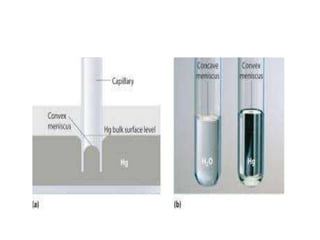
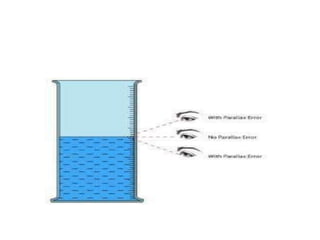
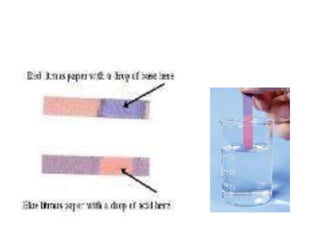
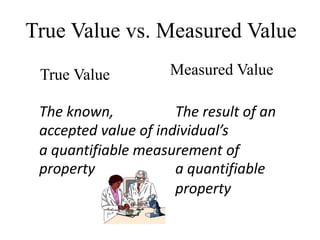
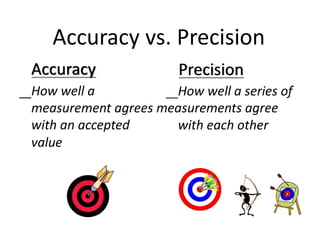
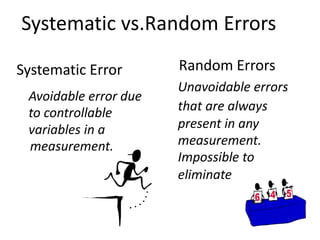
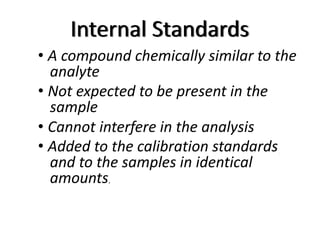
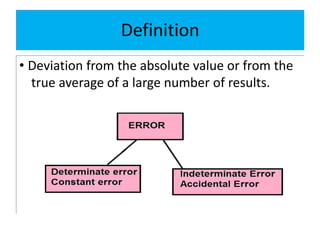
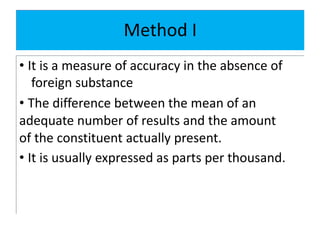
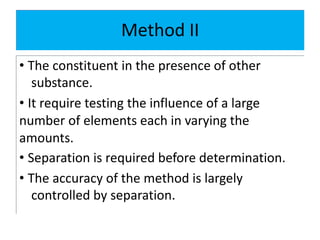
The document discusses various types of errors in pharmaceutical analysis, including determinate, indeterminate, systematic, and random errors, as well as methods to minimize these errors through proper calibration and controls. It contrasts accuracy and precision, highlighting that precision refers to the reproducibility of measurements, while accuracy refers to the closeness to the true value. Additionally, concepts of quality assurance and quality control, as well as the use of internal standards in analysis, are explained.








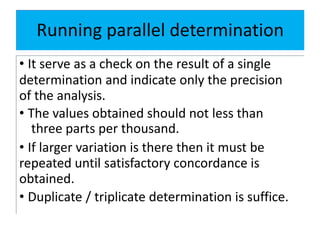
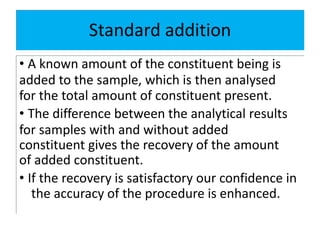

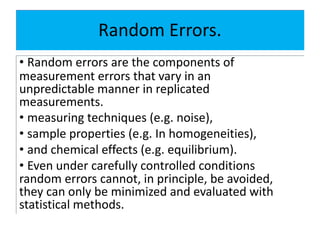
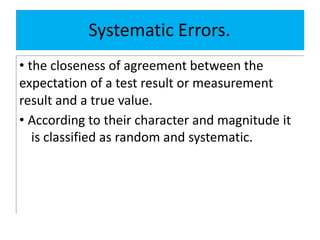

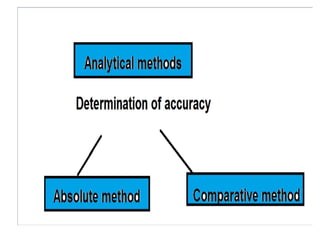
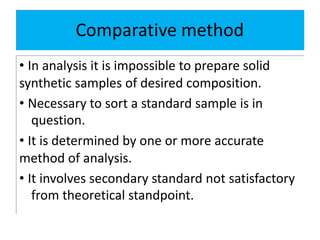
![• It is useful in applied analysis.
• If fundamentally different methods of analysis
for a given constituent [ gravimetric,
titrimetric and spectrometric]. The agreement
between at least two methods of essentially
different character can usually be accepted as
indicating the absence of an appreciable
determinate error .](https://image.slidesharecdn.com/errors-pharmanalysis-1-200405045017/85/Errors-pharmaceutical-analysis-1-33-320.jpg)