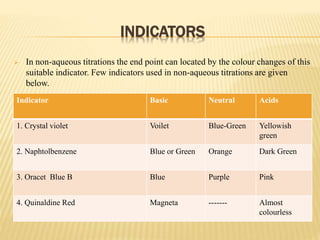
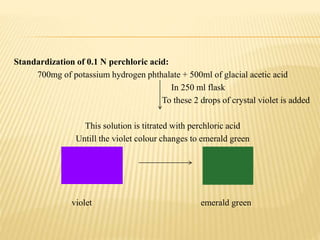
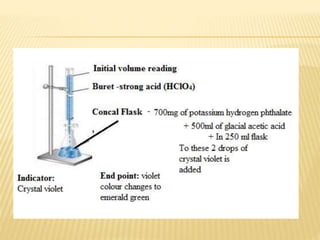
The document discusses non-aqueous titrations, emphasizing their importance in pharmaceutical chemistry for estimating weakly acidic and basic substances using non-aqueous solvents. It explains various types of solvents, indicators, and methodologies for acidimetry and alkalimetry, detailing practical applications such as estimating sodium benzoate and ephedrine hydrochloride. The conclusion summarizes the significance of non-aqueous titrations in simplifying the analysis of substances that are not water-soluble.