Embed presentation
Download to read offline



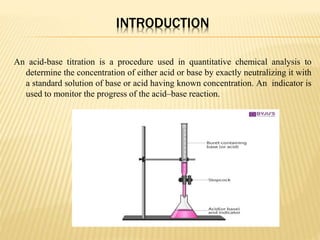



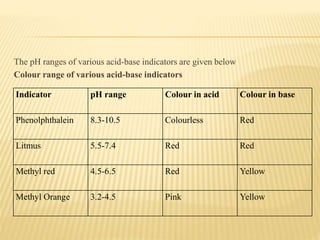
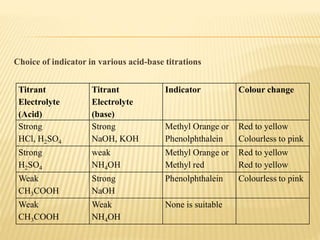



This document discusses acid-base titrations, which are used in quantitative analysis to determine the concentration of an acid or base by neutralizing it with a standard solution of known concentration. Alkalimetry and acidimetry involve titrating a base or acid, respectively, with a standard acid or base. Indicators are used to monitor the reaction and change color at the equivalence point. Common indicators and their pH ranges are identified. The choice of indicator depends on whether the titrant and titrated substances are strong or weak acids and bases.











