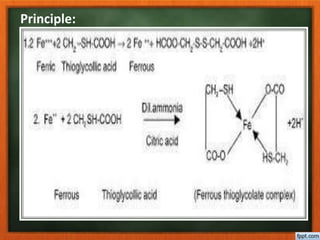
This document describes the limit test for iron according to the Indian Pharmacopoeia. The test involves comparing the color produced by reacting a sample with thioglycolic acid in an ammonical citrate buffer to the color produced by a standard iron solution under the same conditions. If the color produced by the sample is less than the standard, it passes the limit test for iron. If the color is greater than the standard, it fails the limit test. The test is sensitive and uses citric acid to eliminate interference from other metal cations.
















![Sample I [ Pass Sample ] :
• Observation:
• The Colour produced in the test solution is
lesser than standard solution.
• Inference:
• The given substance passes the limit test for
Iron as per Indian pharmacopoeia when
compared with that of a standard substance.](https://image.slidesharecdn.com/4-170808044829/85/4-limit-test-for-iron-18-320.jpg)
![Sample II [ Fail Sample ] :
• Observation:
• The Colour produced in the test solution is
more than standard solution.
• Inference:
• The given substance fails the limit test for
Iron as per Indian pharmacopoeia when
compared with that of a standard substance.](https://image.slidesharecdn.com/4-170808044829/85/4-limit-test-for-iron-19-320.jpg)
