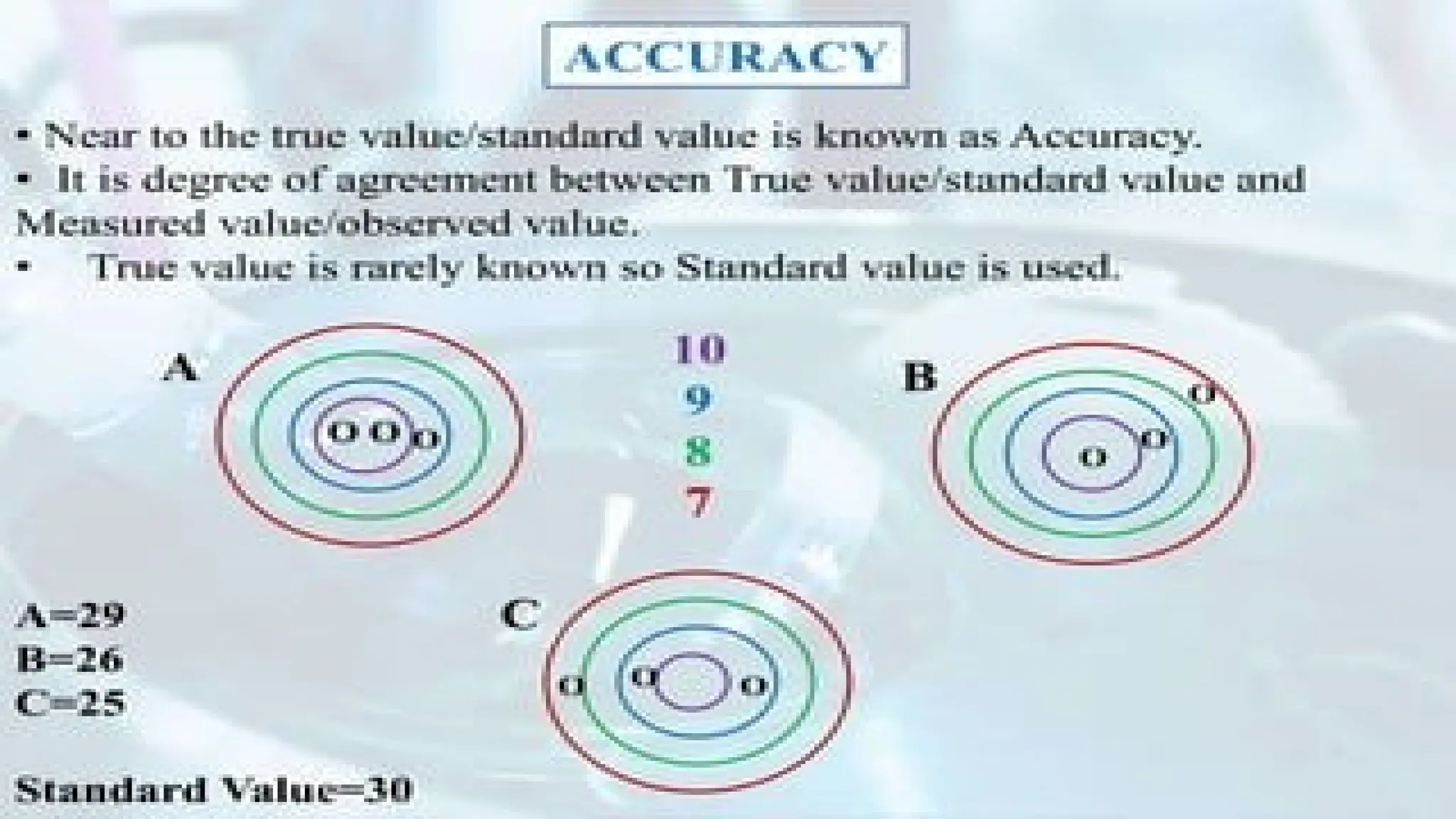
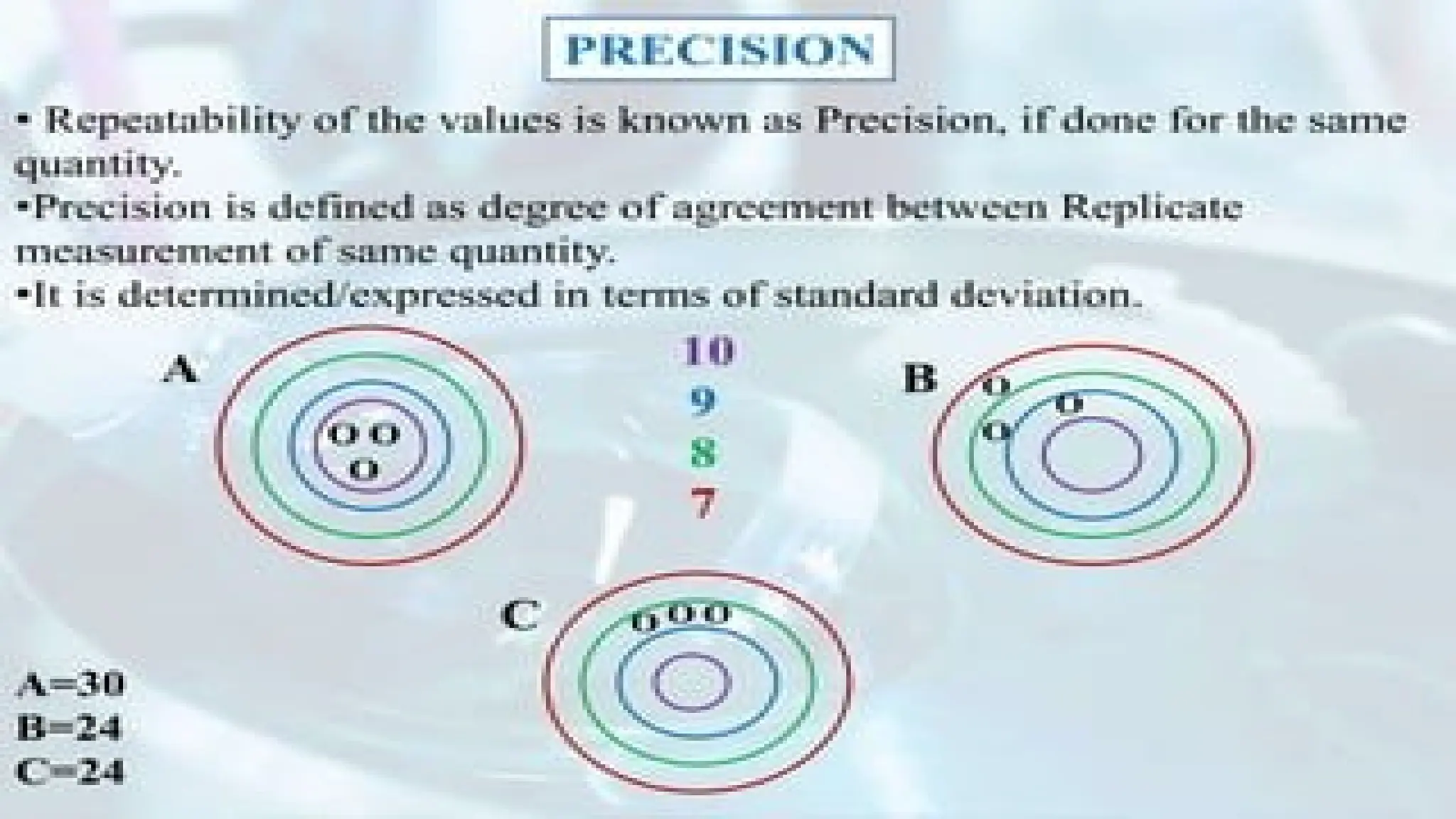
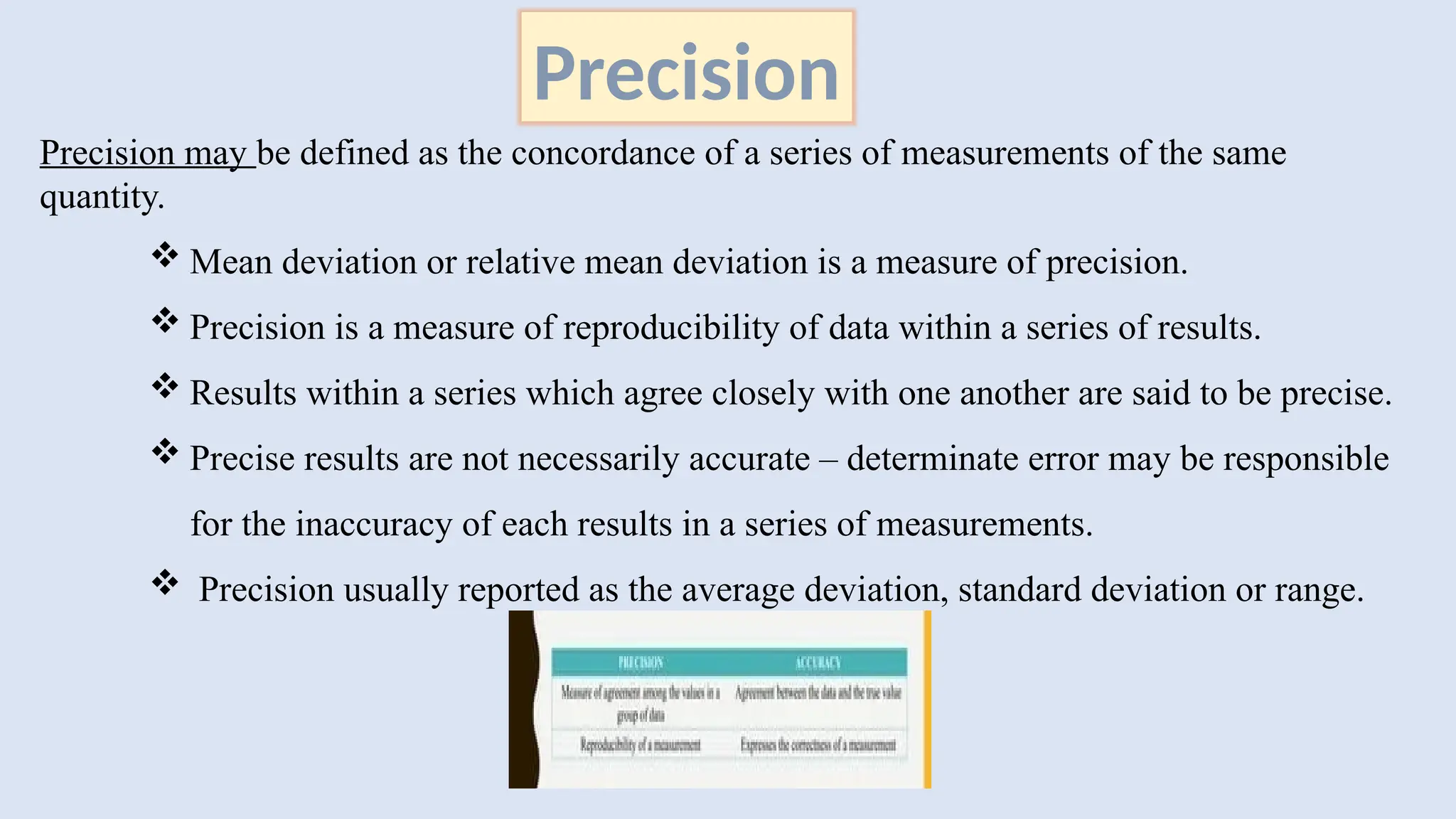
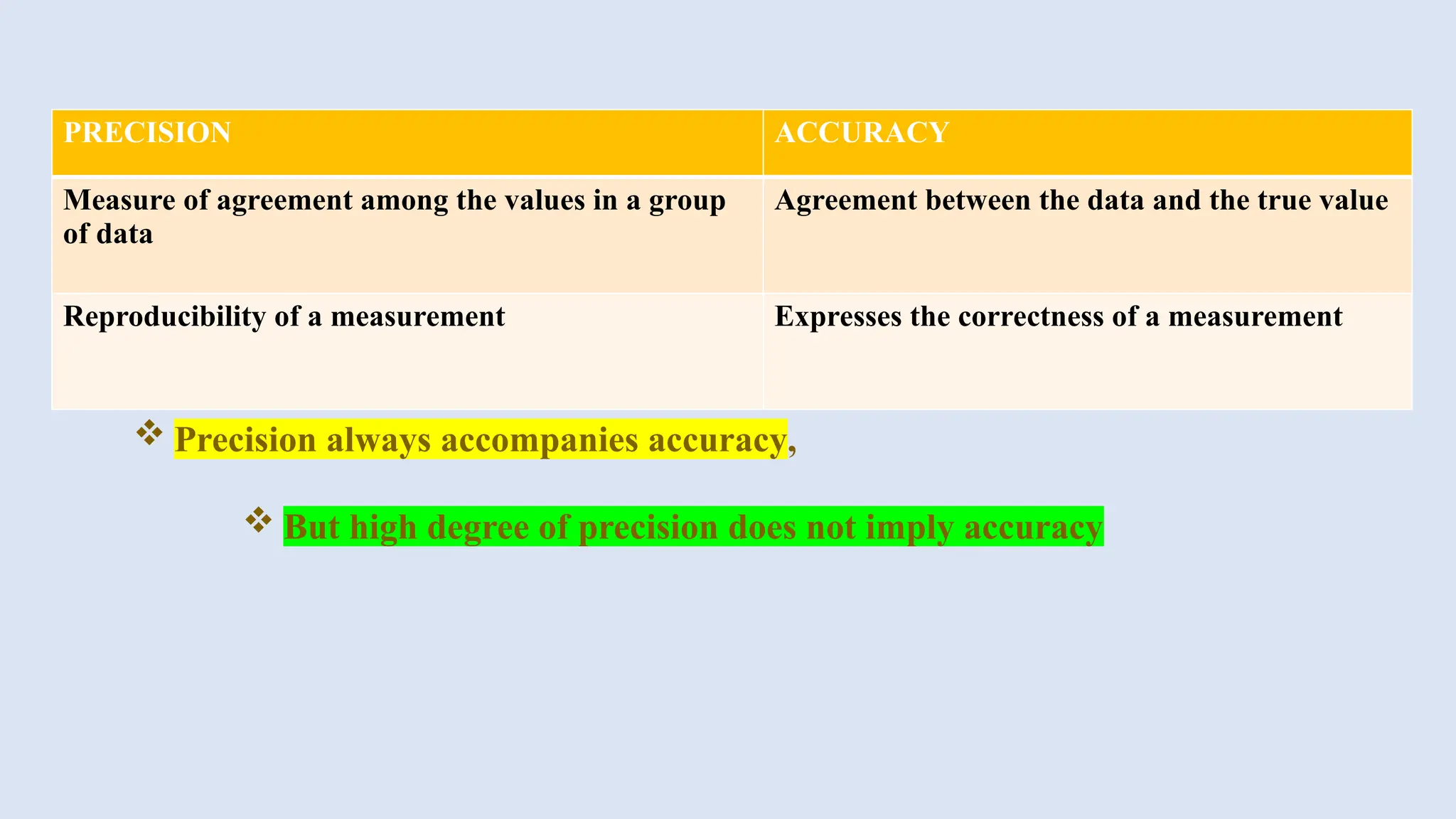
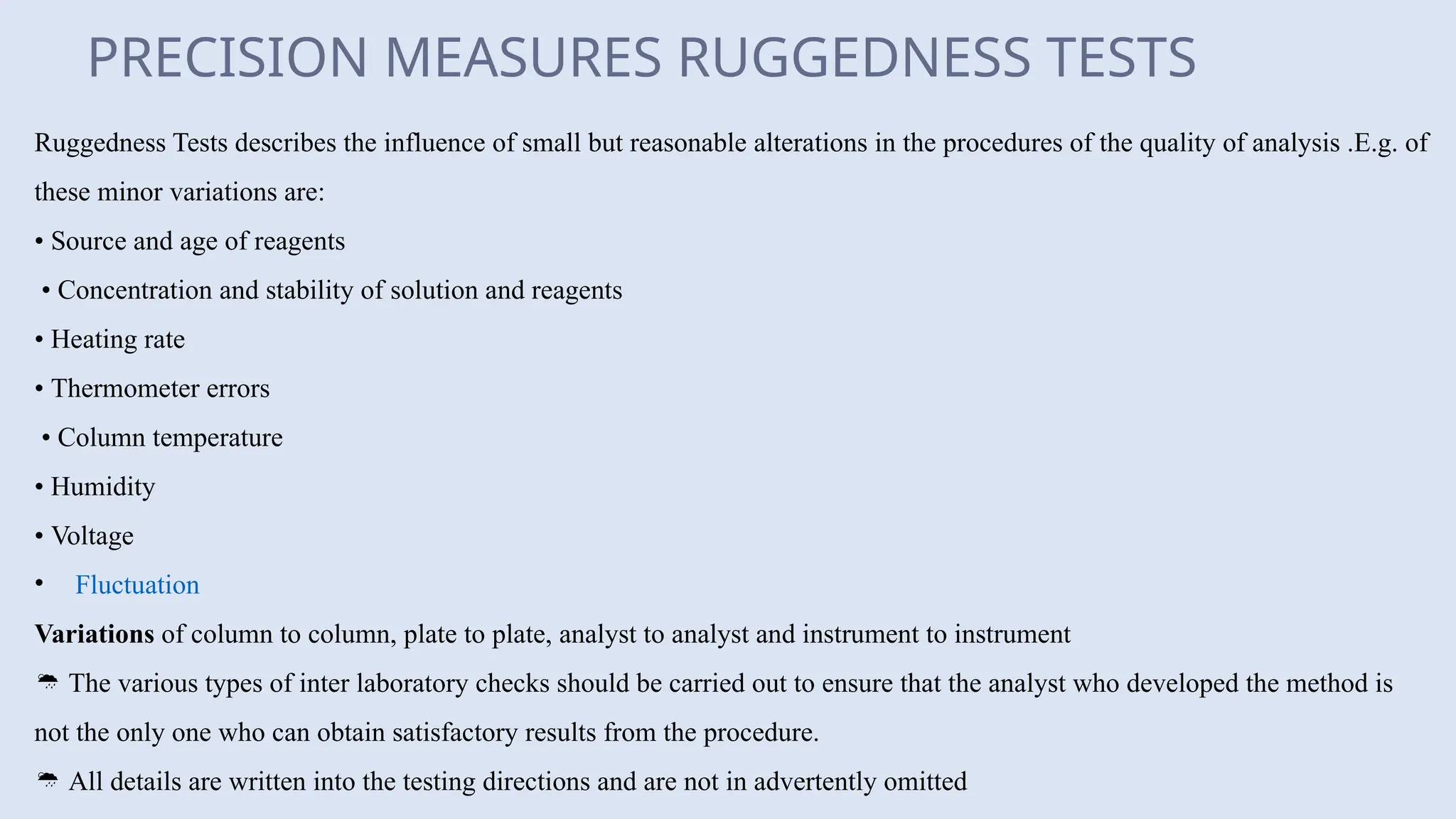
The document discusses various types of errors in pharmaceutical analysis, including the difference between true and measured results, and categorizes errors into determinate (systematic) and indeterminate (random) errors. Factors influencing errors include human sources, instrument quality, experimental conditions, and improper methodology. The document also outlines methods for improving accuracy and precision in analytical measurements, such as calibration, blank determination, and adherence to significant figures and rounding rules.