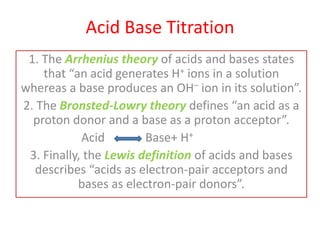
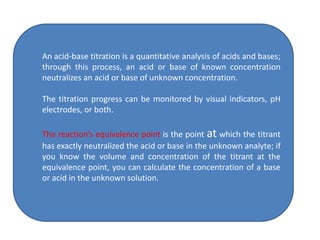
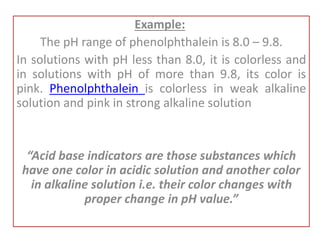
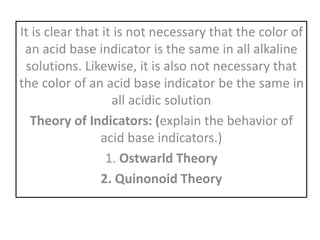
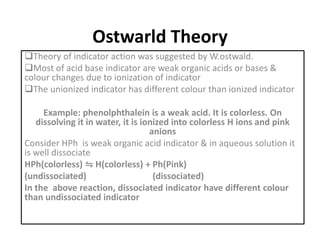
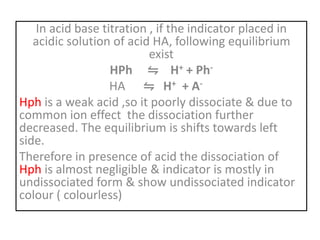
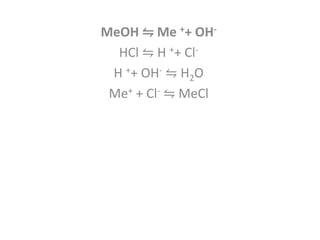
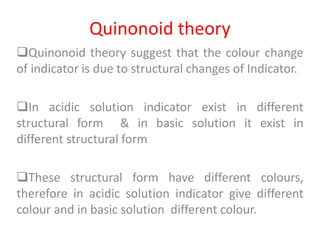
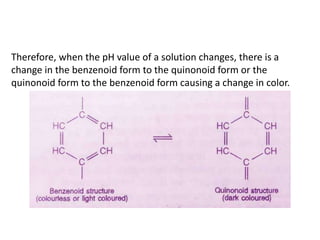
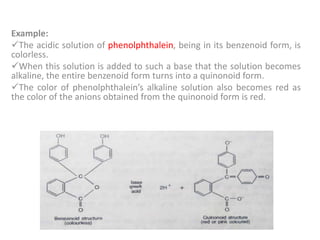
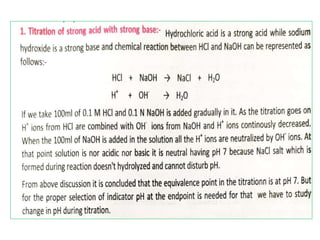
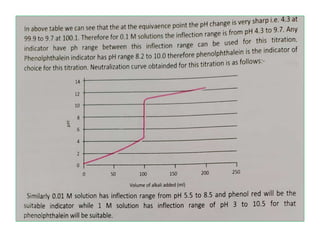
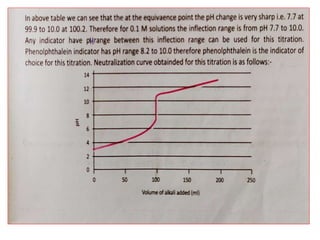
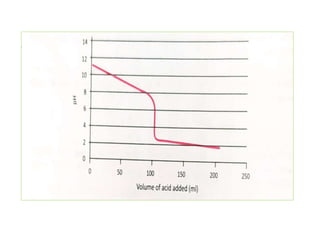
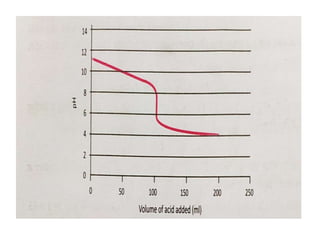
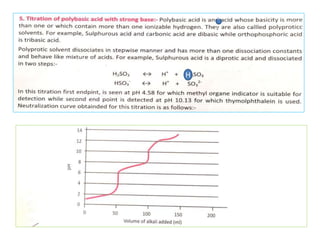
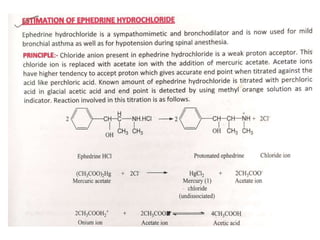
This document discusses acid-base titration and indicators. It defines acid-base titration as a quantitative analysis that involves neutralizing an acid or base of unknown concentration with an acid or base of known concentration. The equivalence point occurs when the titrant has exactly neutralized the analyte and allows calculating the concentration of the unknown acid or base. It also describes the Ostwald and quinonoid theories for how acid-base indicators work by changing color depending on their state of ionization or structural form under acidic or basic conditions.