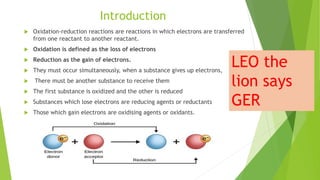
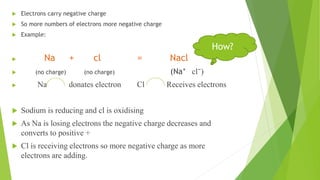
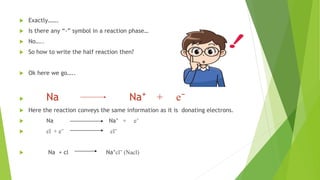
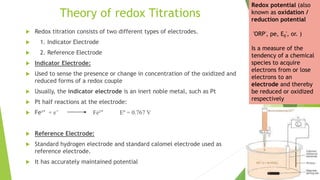
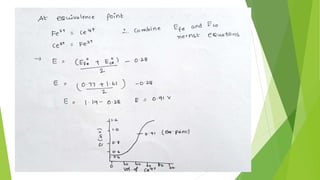
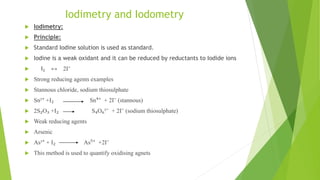
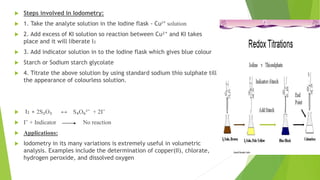
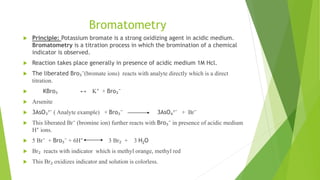
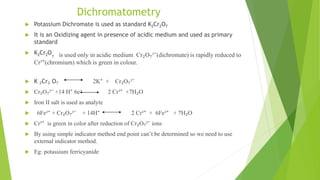
The document outlines redox titrations, focusing on oxidation-reduction reactions, half reactions, and the roles of oxidizing and reducing agents. It describes various titration methods including iodimetry, bromatometry, and dichromatometry, as well as the use of redox indicators and electrodes in these processes. Additionally, the document explains the concept of equivalent weight and the application of different electrodes in measuring redox potentials.