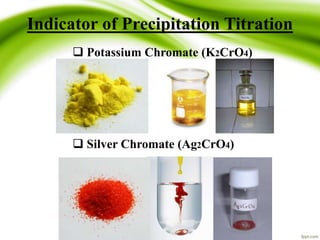
This document discusses precipitation titration, which involves the formation of an insoluble precipitate during titration. It describes the Mohr, Volhard, and Fajans methods for detecting the endpoint of precipitation titrations using different indicators like chromate, iron, and fluorescein. The Volhard method, which detects the endpoint potentiometrically by titrating excess silver with thiocyanate, is highlighted as being widely used. Limitations and ways to overcome problems of precipitation titration are also outlined.