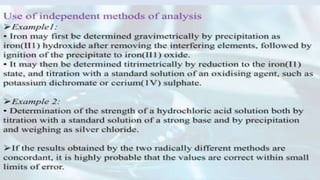
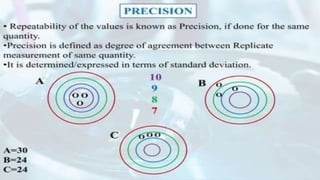
The document outlines various types of errors in pharmaceutical analysis, emphasizing their sources, including human factors, instrument quality, and experimental conditions. It categorizes errors into determinate (systematic) and indeterminate (random), detailing how each type affects accuracy and precision. Additionally, it discusses methods to minimize errors, such as calibration, control determinations, and understanding significant figures in measurements.