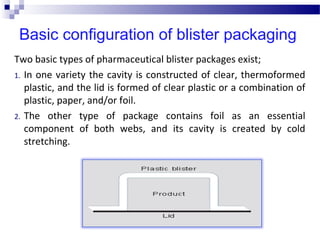
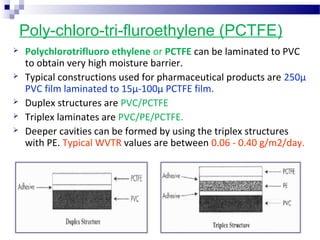
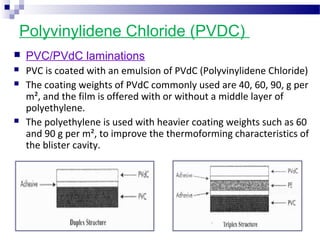
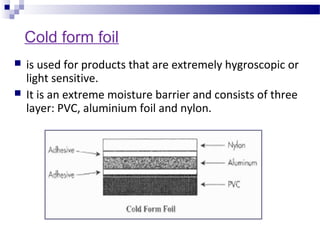
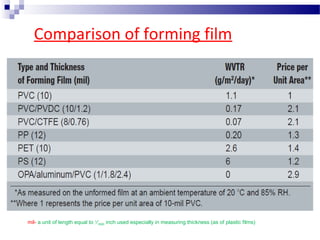
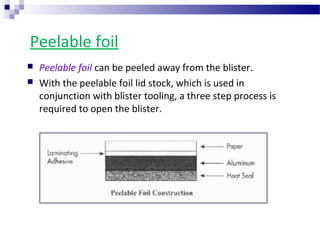
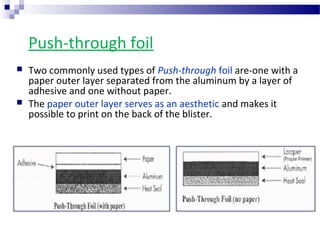
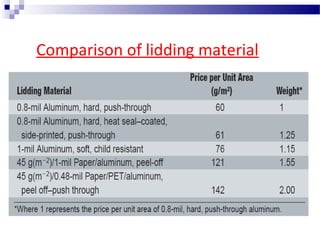
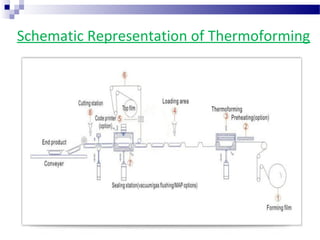
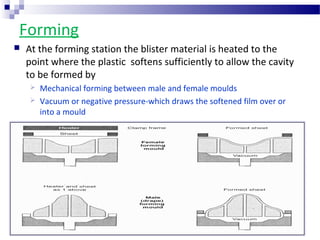
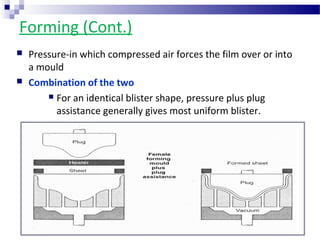
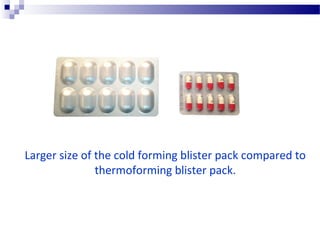
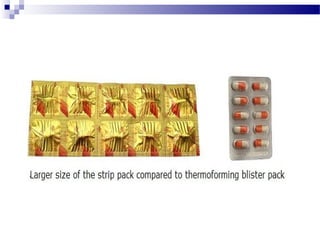
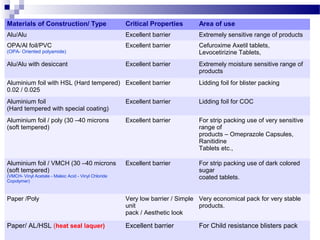
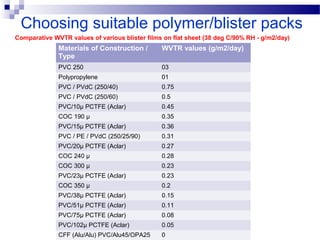
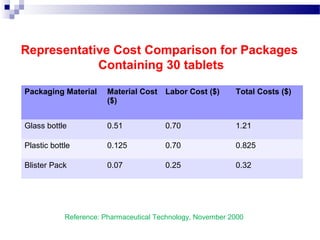
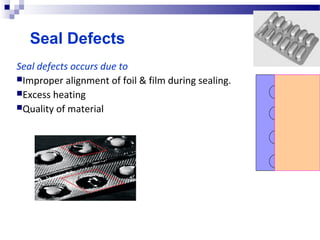
The document provides an extensive overview of blister and strip packaging, detailing the structure, advantages, and production methods of blister packs commonly used in pharmaceuticals and consumer goods. Key components include forming films, lidding materials, heat seal coatings, and printing inks, each with specific properties that ensure product integrity and compliance with safety standards. Additionally, it outlines the methods of production such as thermoforming and cold forming, highlighting the benefits and limitations of each type of blister packaging.