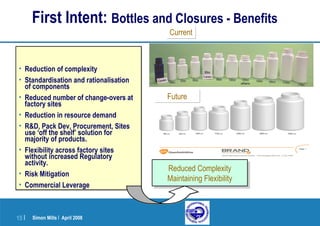
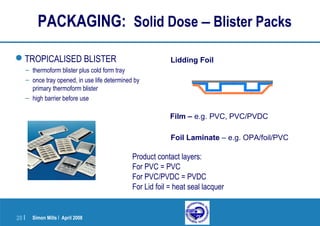
This document provides an overview of a presentation on pharmaceutical packaging and development with a focus on pediatric formulations. It discusses choosing appropriate primary packaging, regulatory considerations, extractables and leachables testing, and packaging development processes. Specific topics covered include blister packs, bottles, closures, barrier properties of materials, desiccants, oxygen and water vapor transmission rates, and first intent guidelines.