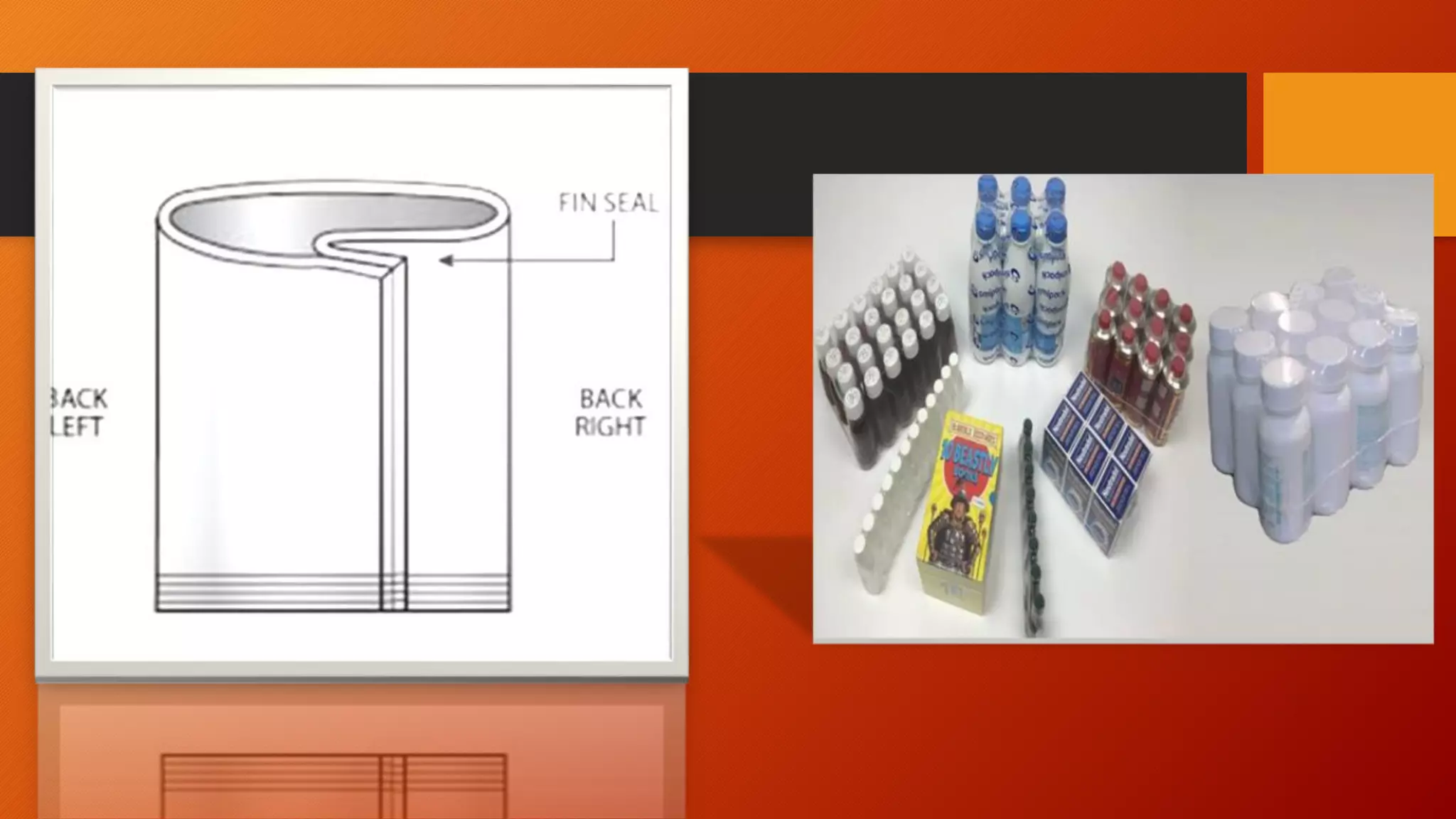
The document discusses plastic packaging materials used for pharmaceutical products. It begins by describing the two main categories of plastics - thermoplastics and thermosets. It then discusses potential interactions between drugs and plastic packaging, including permeation, leaching, sorption, and chemical reactions. Finally, it covers various closure and sealing methods that are approved by the FDA as tamper resistant packaging systems for pharmaceuticals, such as blister packs, bubble packs, foil/plastic pouches, and bottle seals.