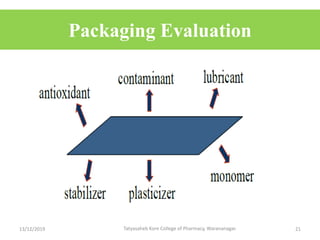
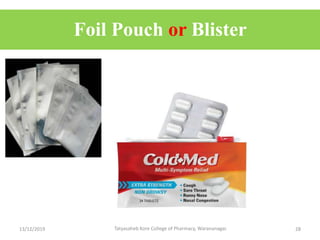
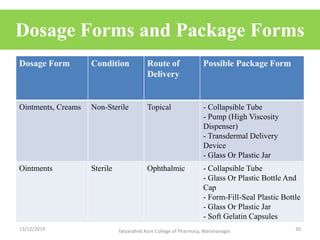
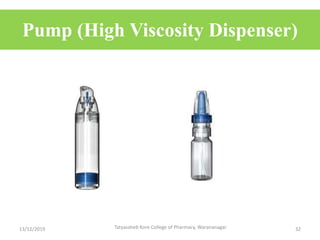
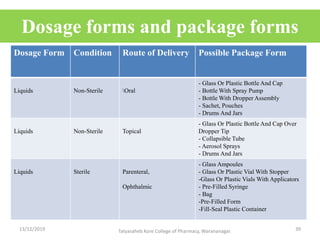
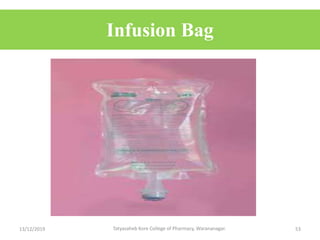
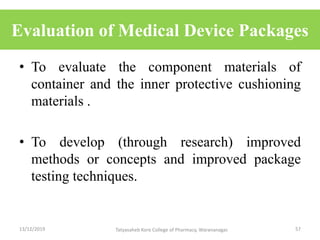
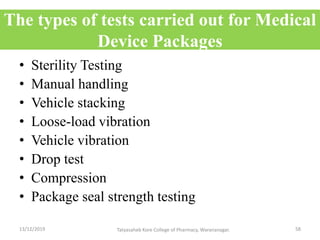
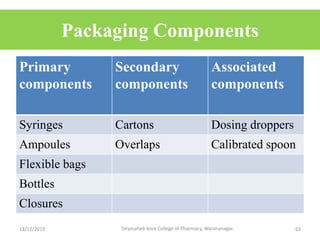
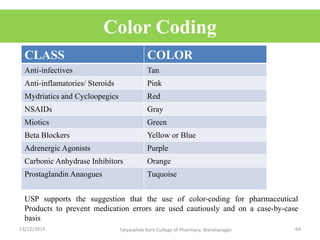
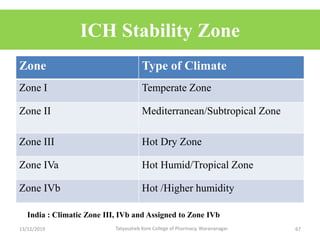
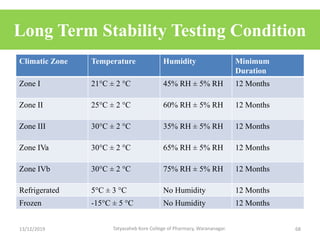
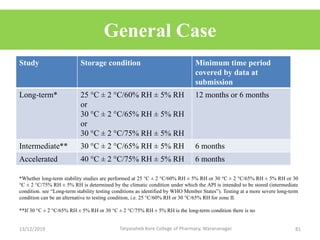
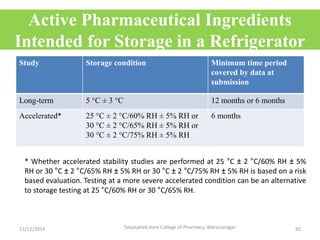
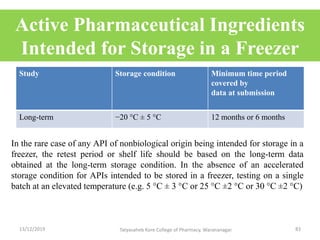
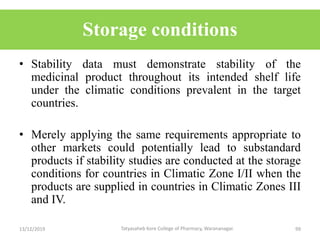
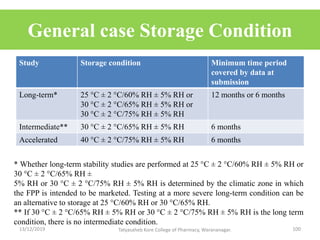
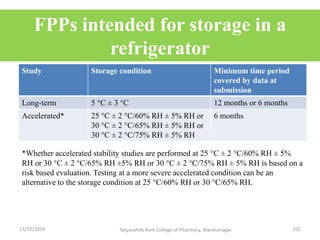
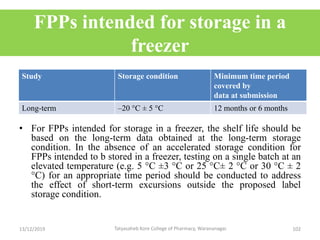
This document discusses packaging and stability requirements for pharmaceuticals. It covers topics such as the purpose of pharmaceutical packaging in protecting drugs and ensuring stability. Various primary and secondary packaging materials are described, including glass, plastic and closures. The document also discusses packaging for different dosage forms like solids, liquids and medical devices. Key considerations for packaging include protection, compatibility, safety and performance. Packaging must meet regulations and standards to ensure drug quality and stability.