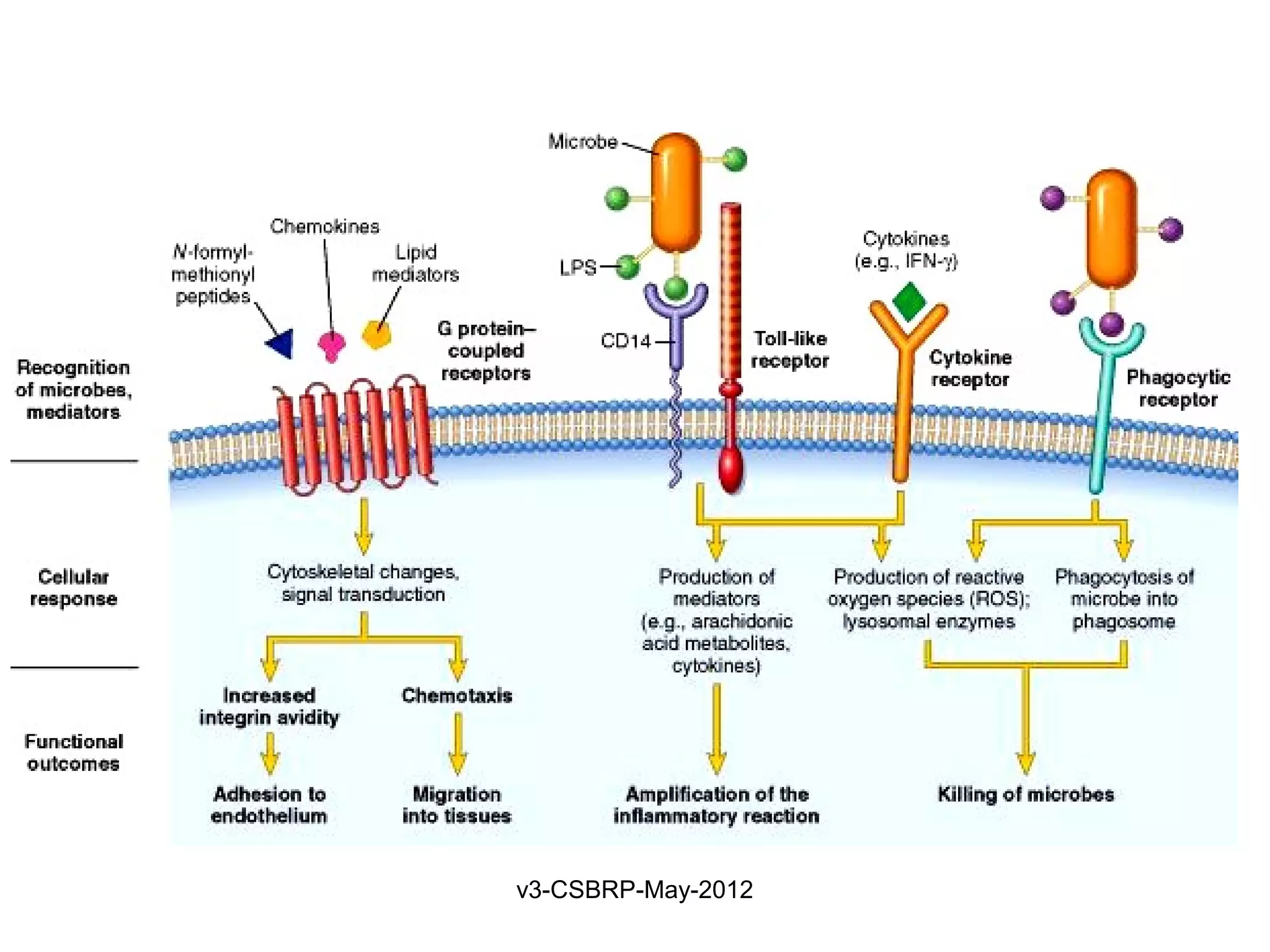
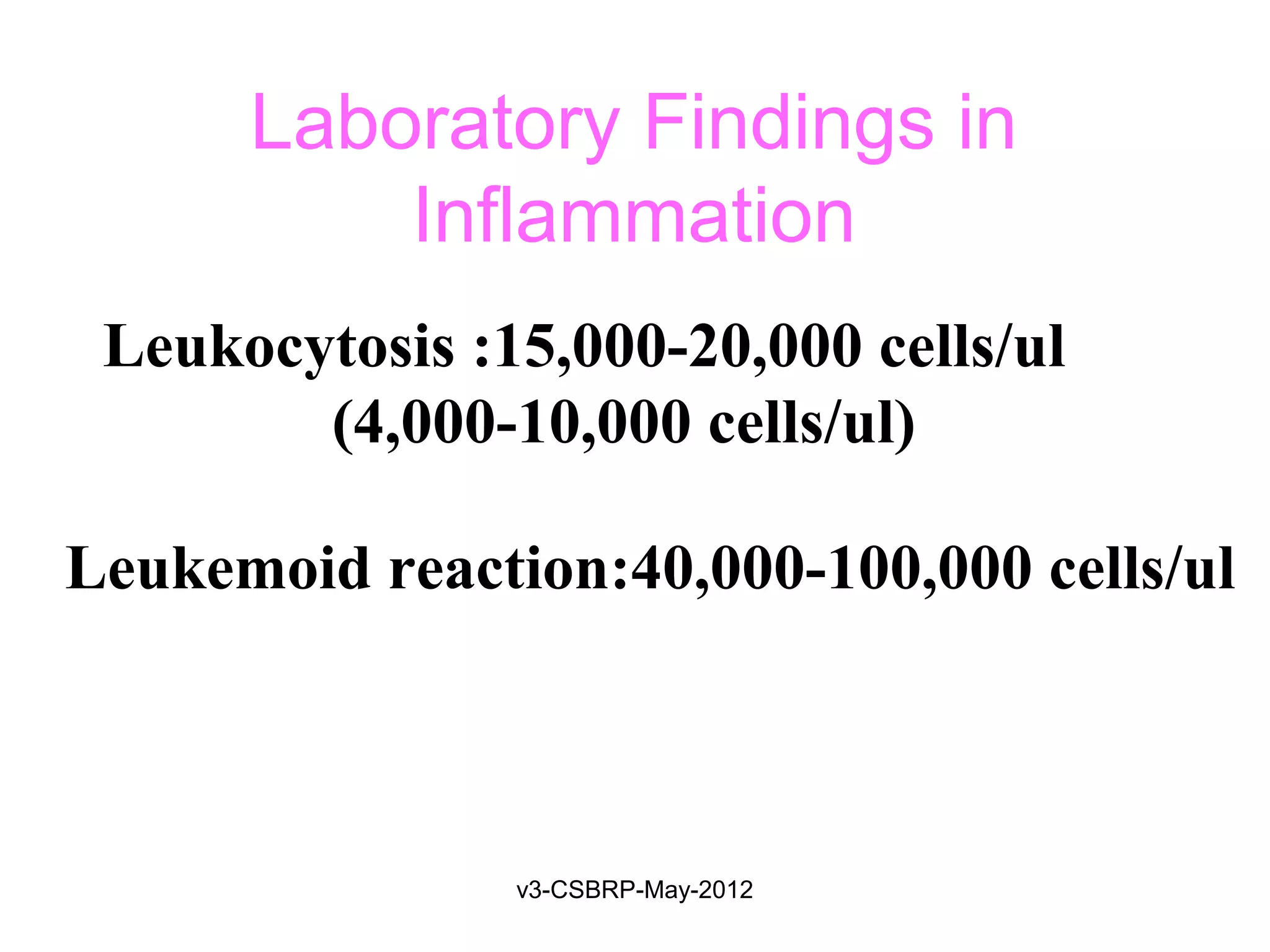
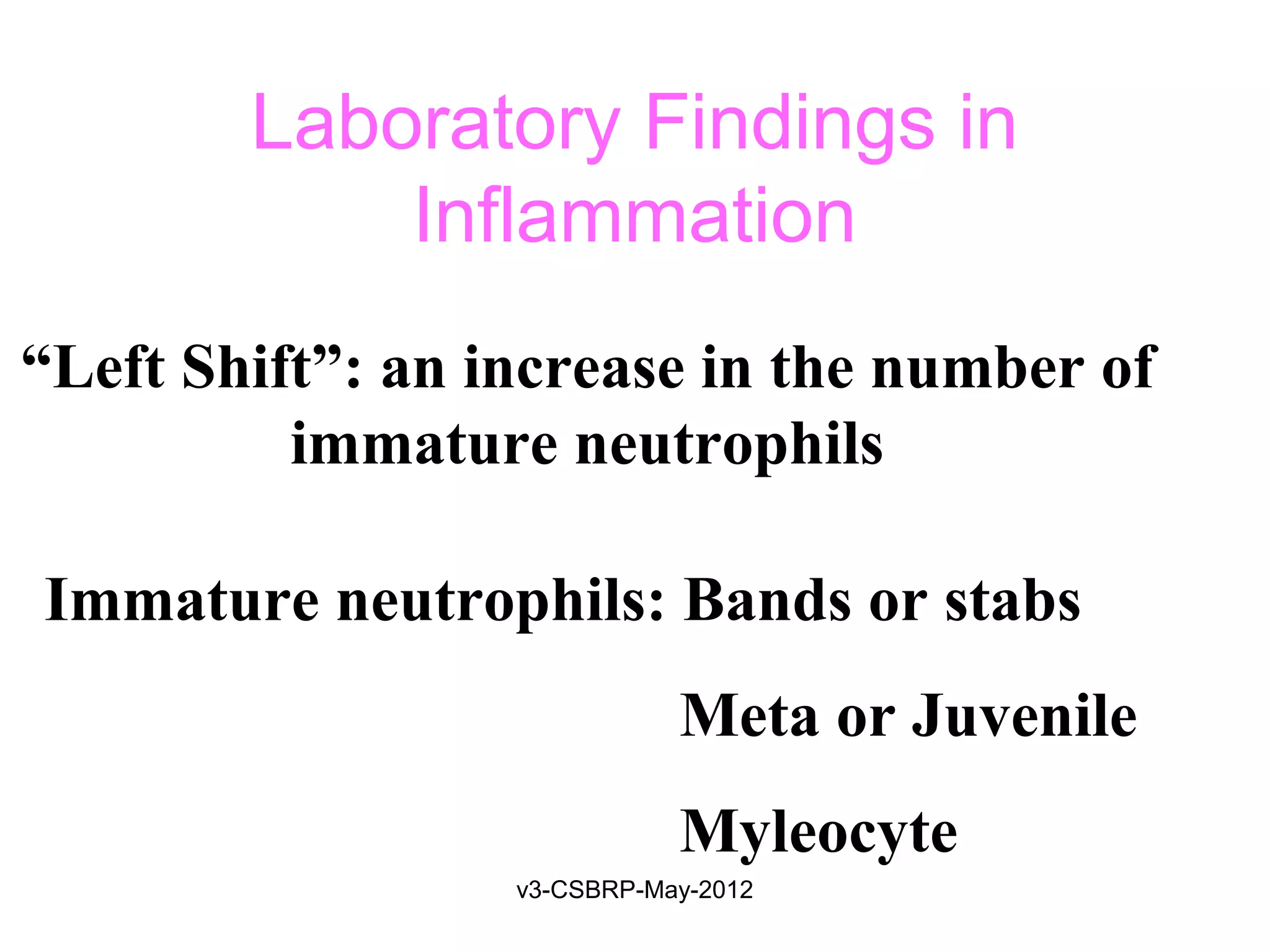
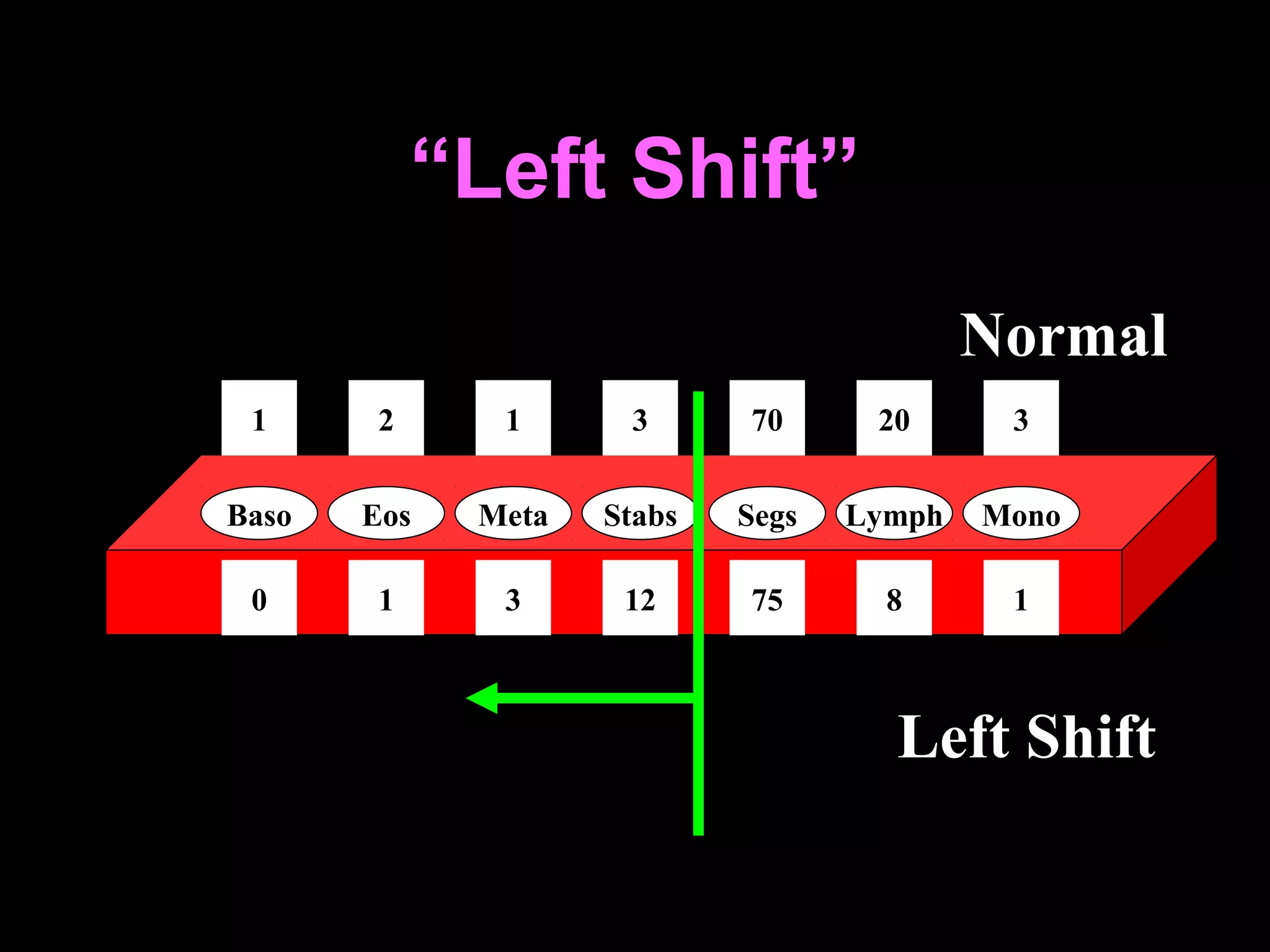
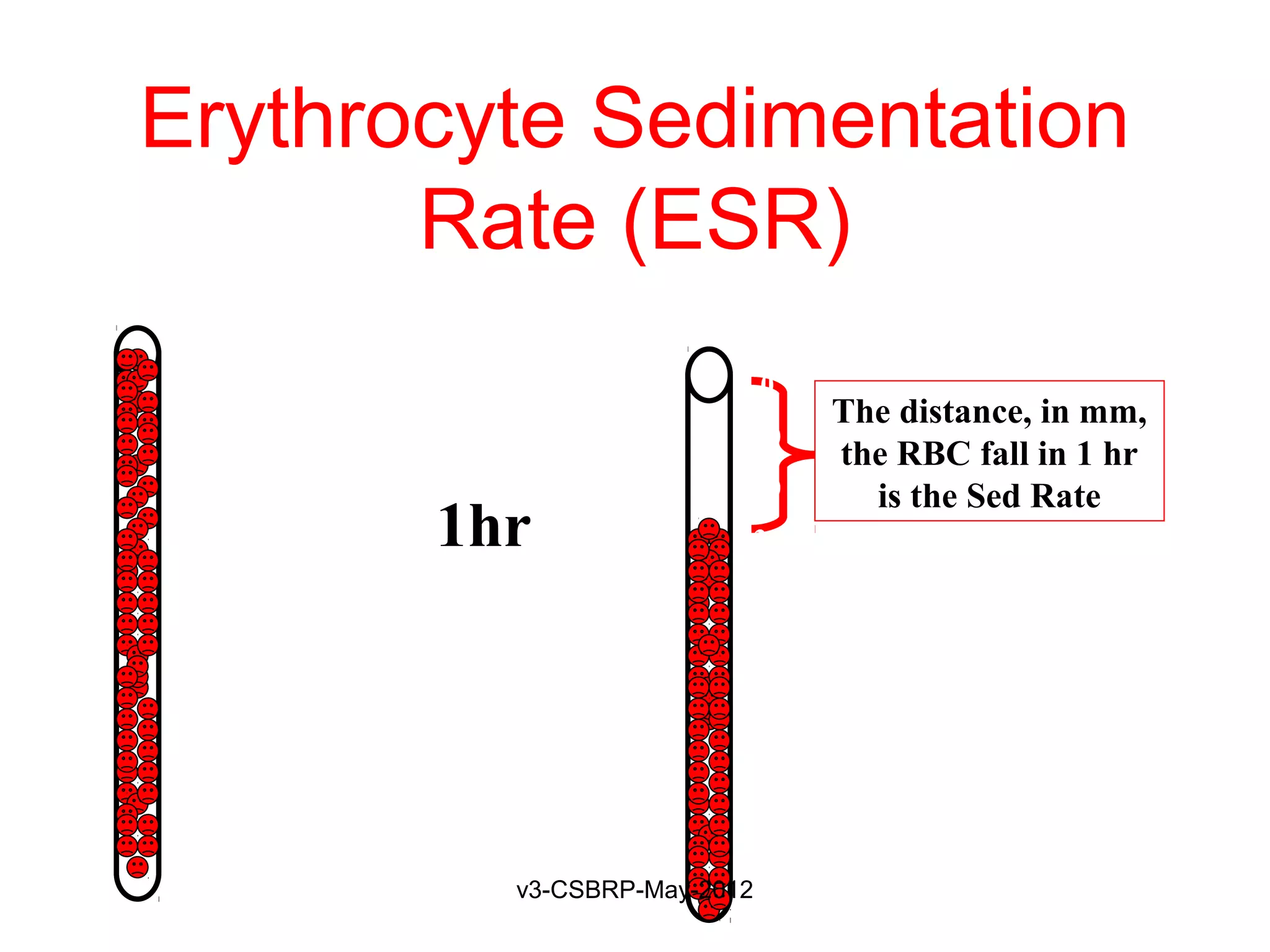
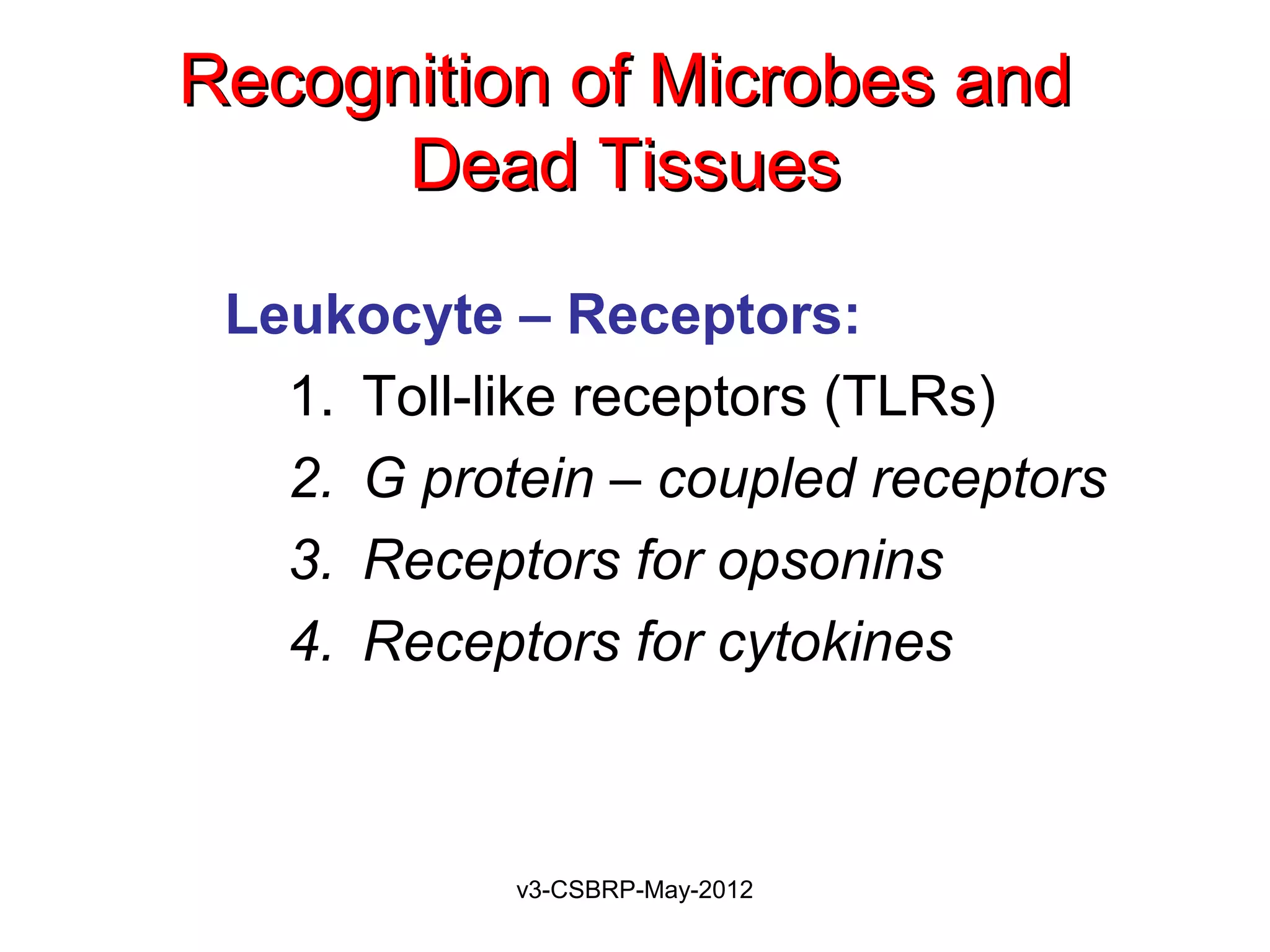
The document discusses leukocyte receptors that recognize microbes and dead tissues. It describes four main classes of leukocyte receptors: Toll-like receptors, G protein-coupled receptors, receptors for opsonins, and receptors for cytokines. Toll-like receptors bind to microbial products and mediate cellular responses. G protein-coupled receptors recognize bacterial peptides and chemokines. Receptors for opsonins promote phagocytosis by binding immunoglobulins, complement proteins, and lectins. Receptors for cytokines such as interferon-gamma activate leukocytes.
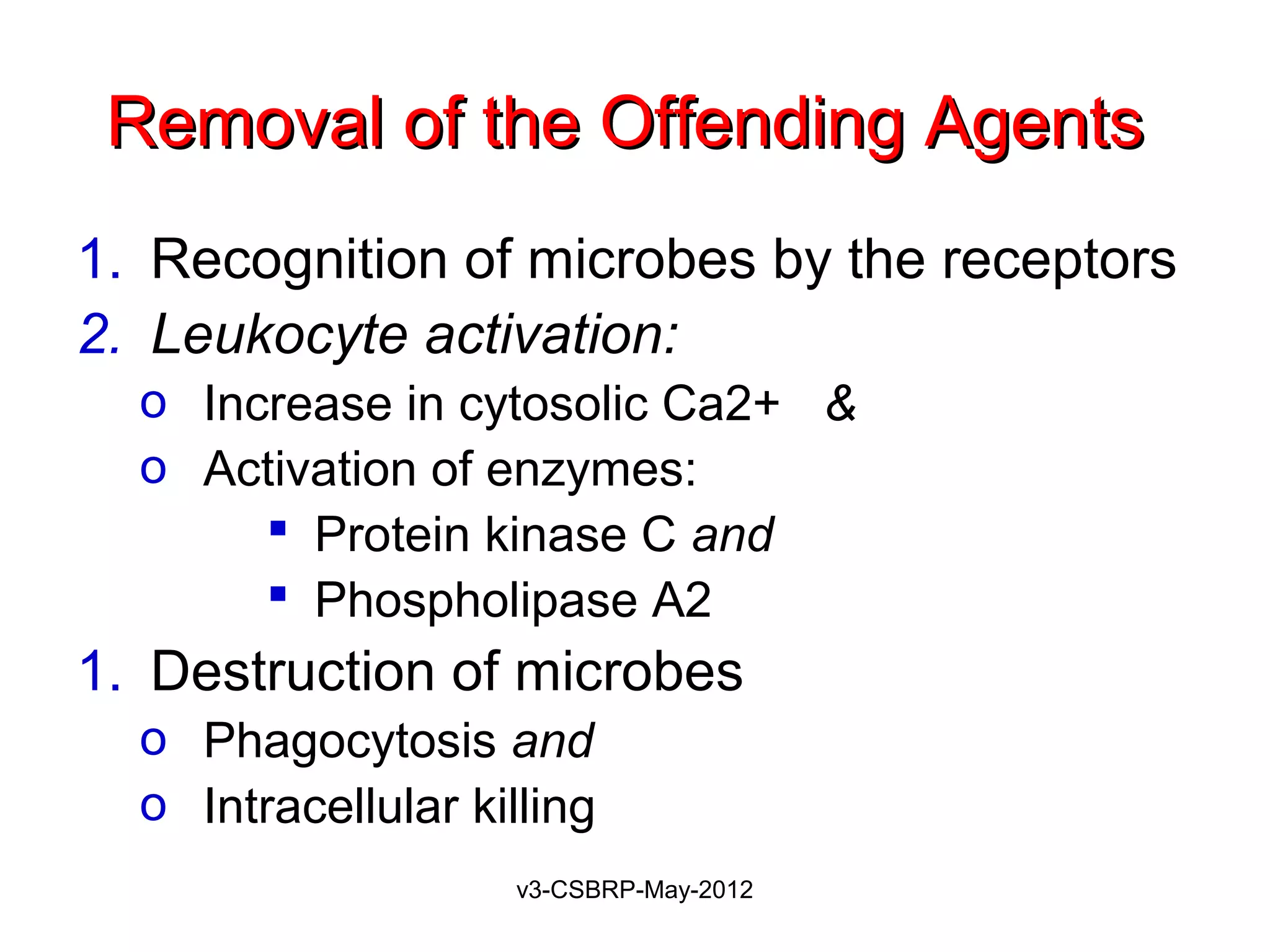
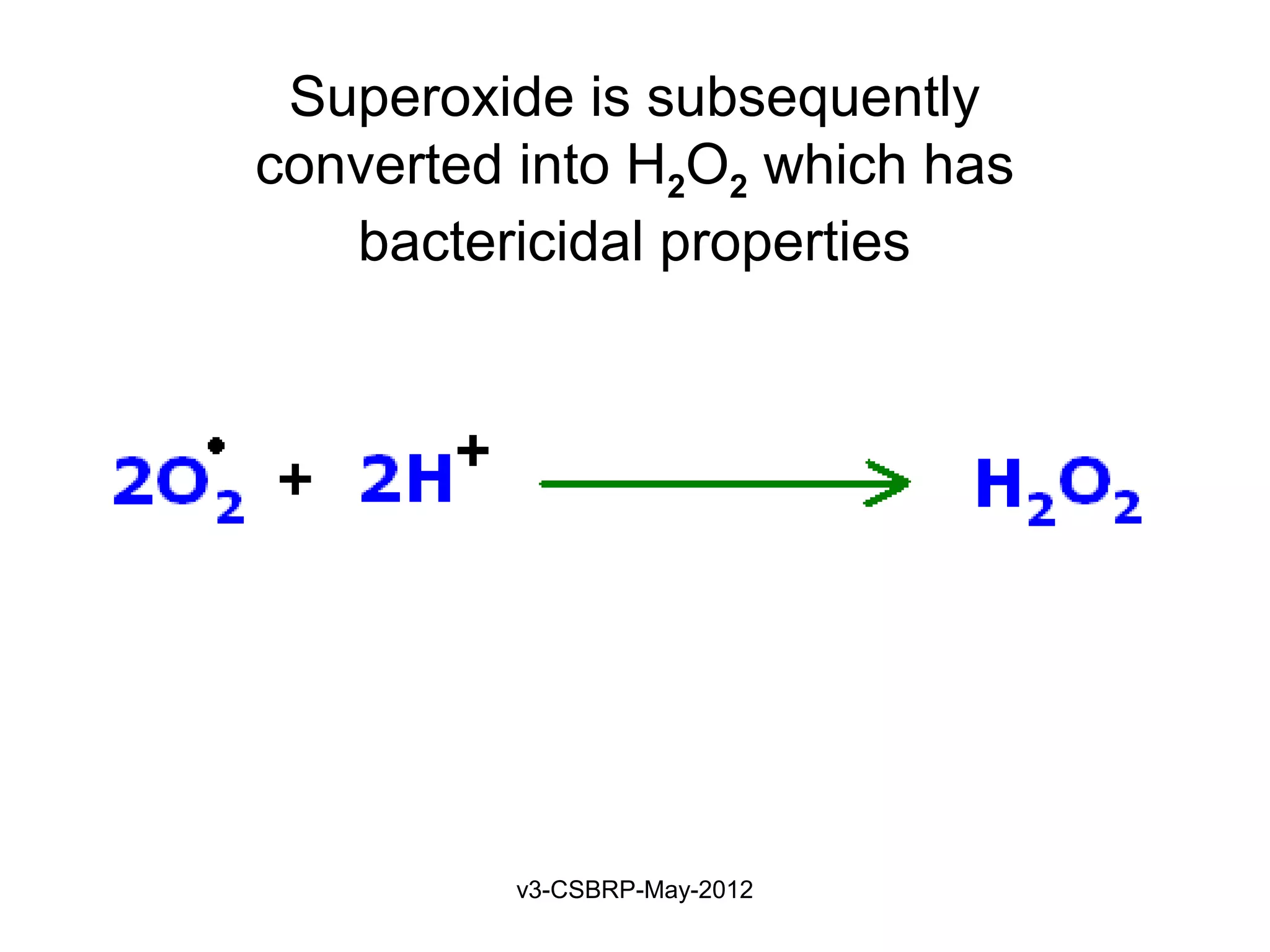
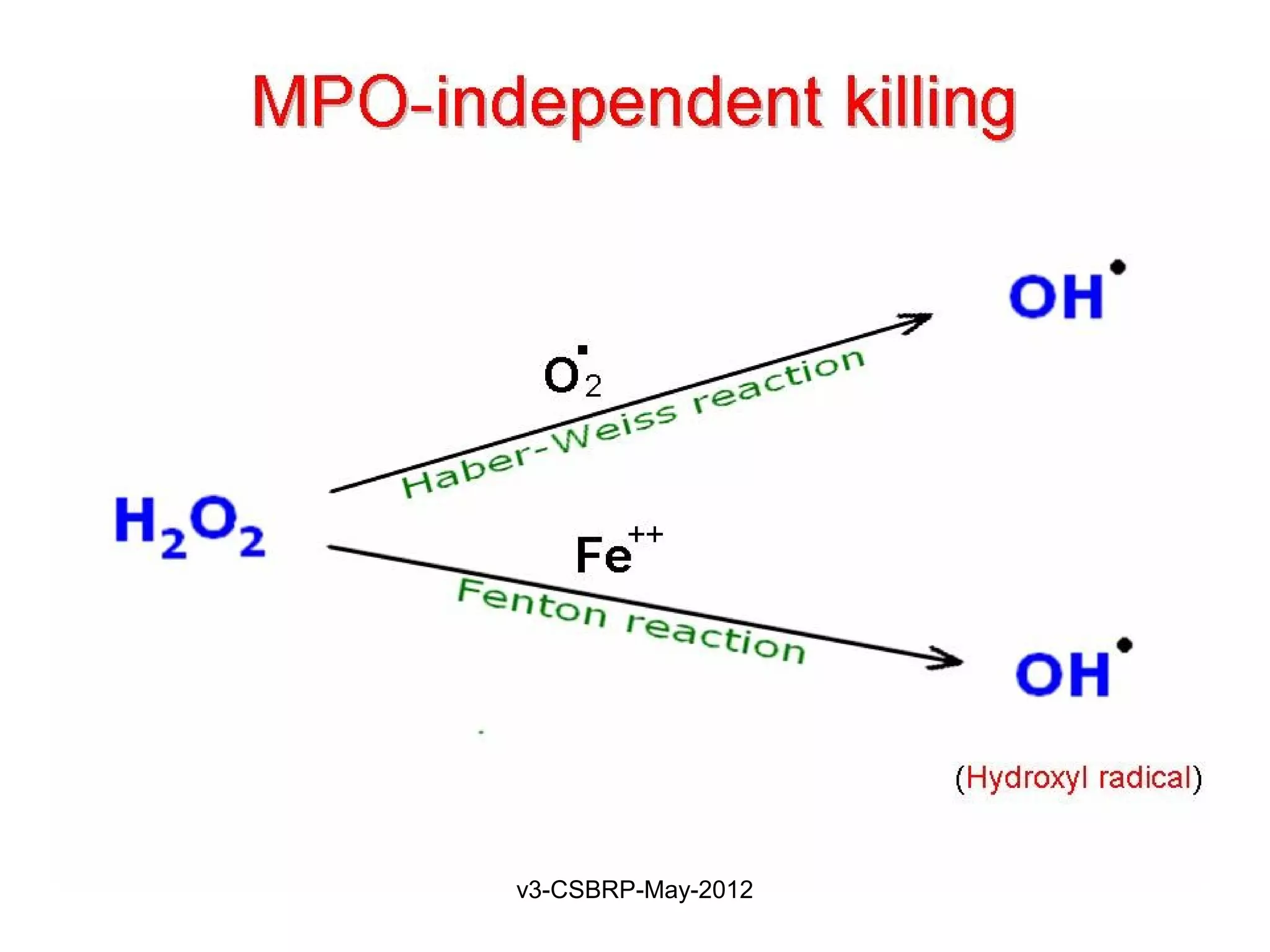
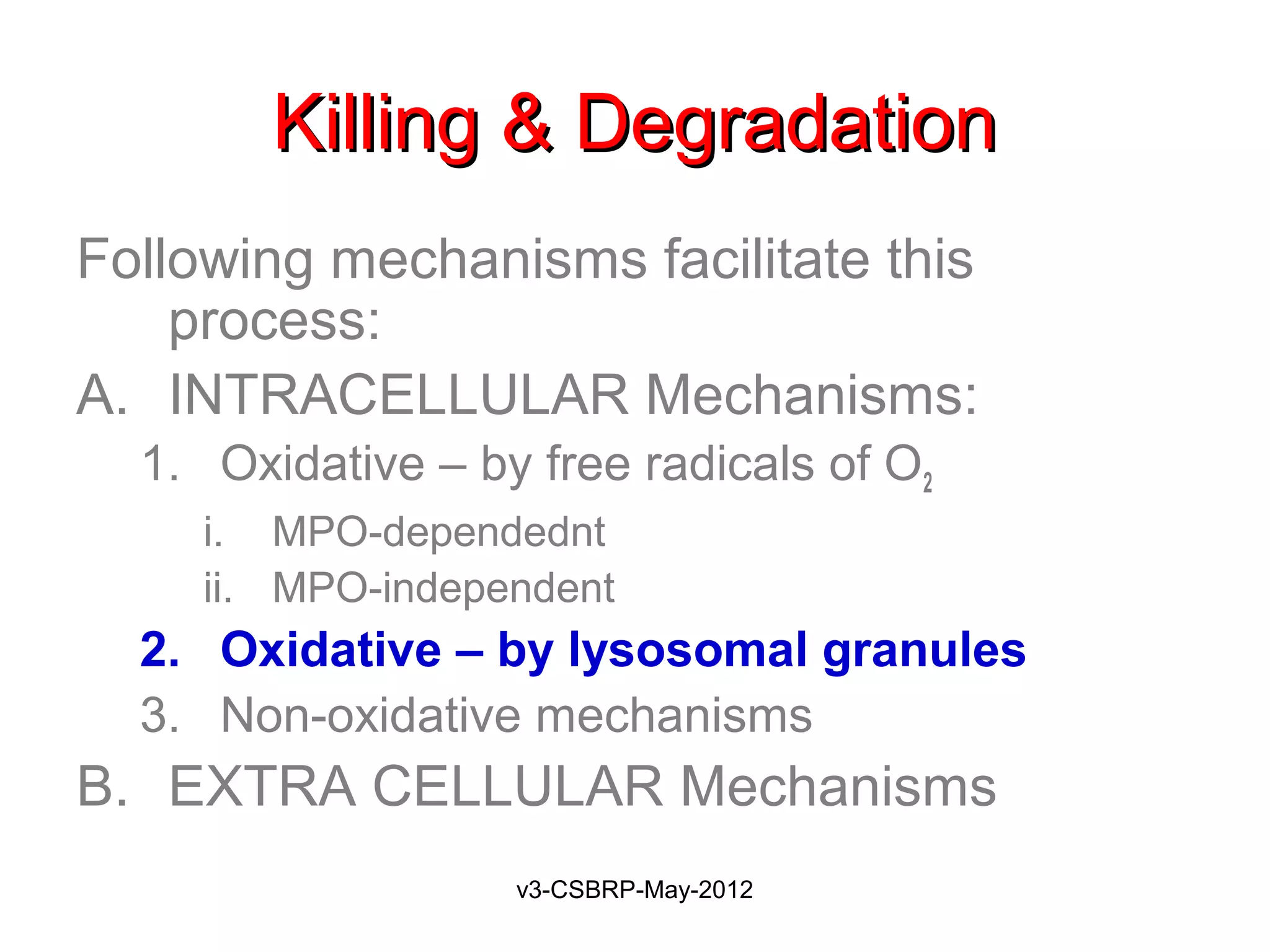
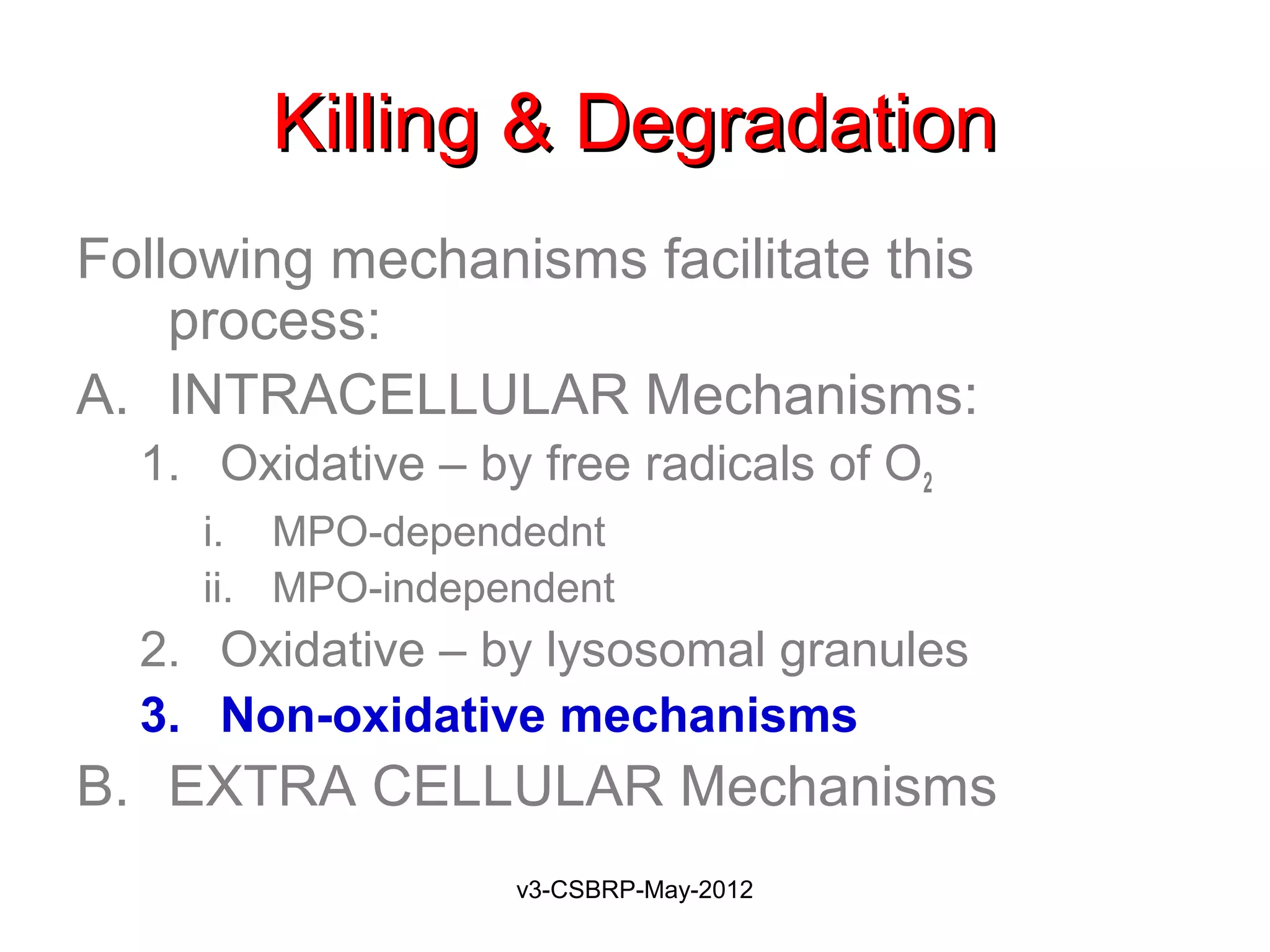
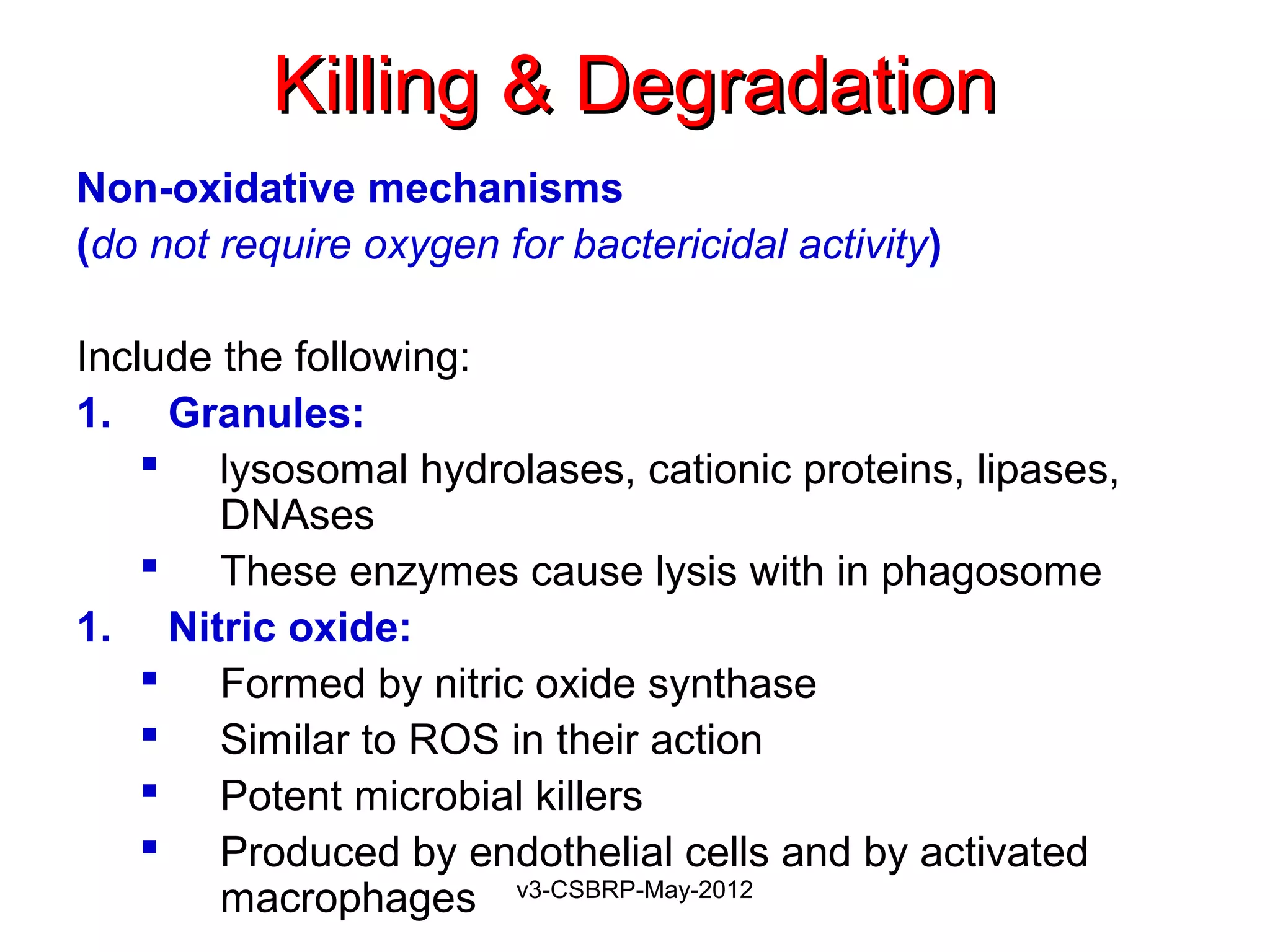
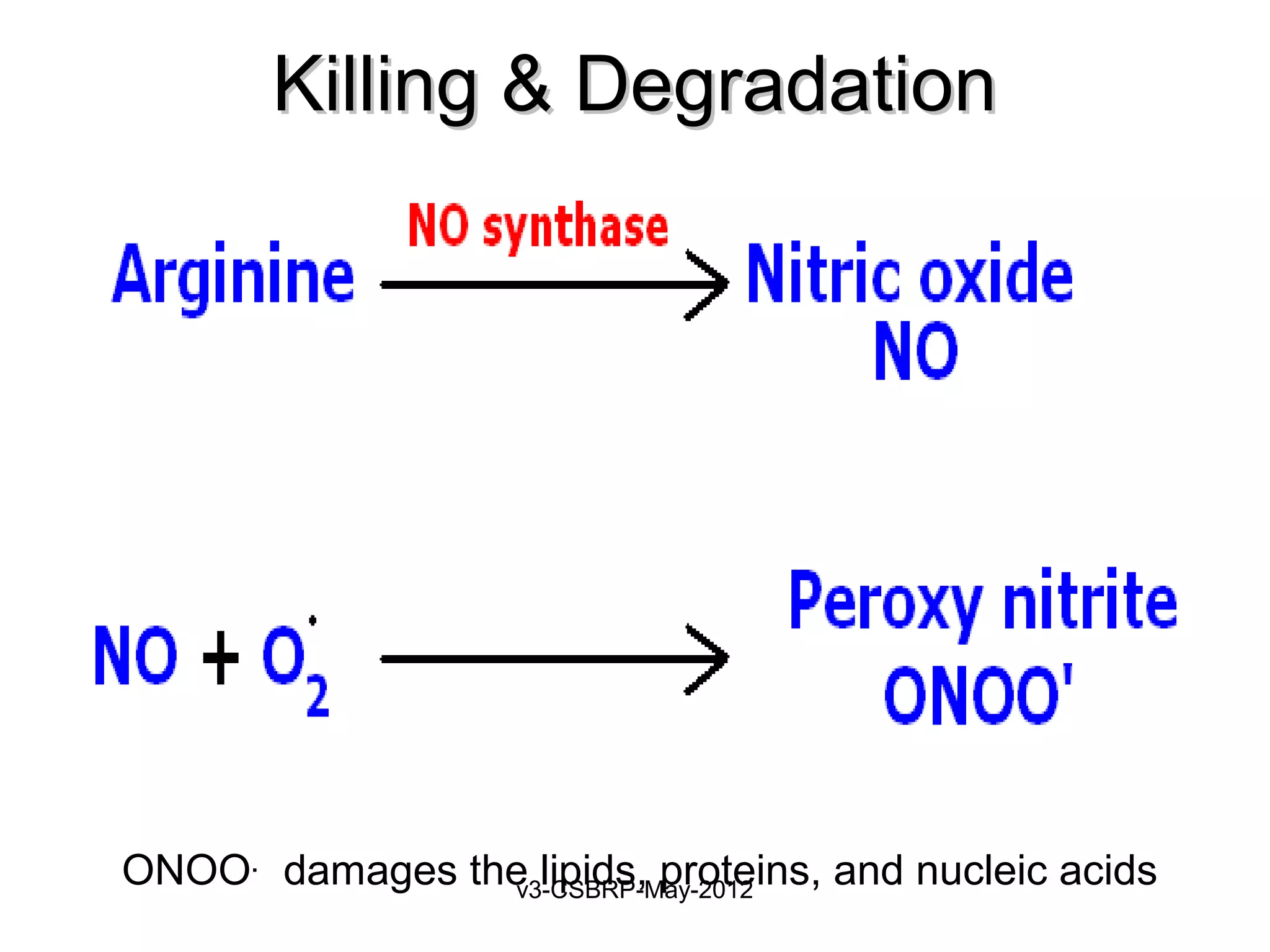
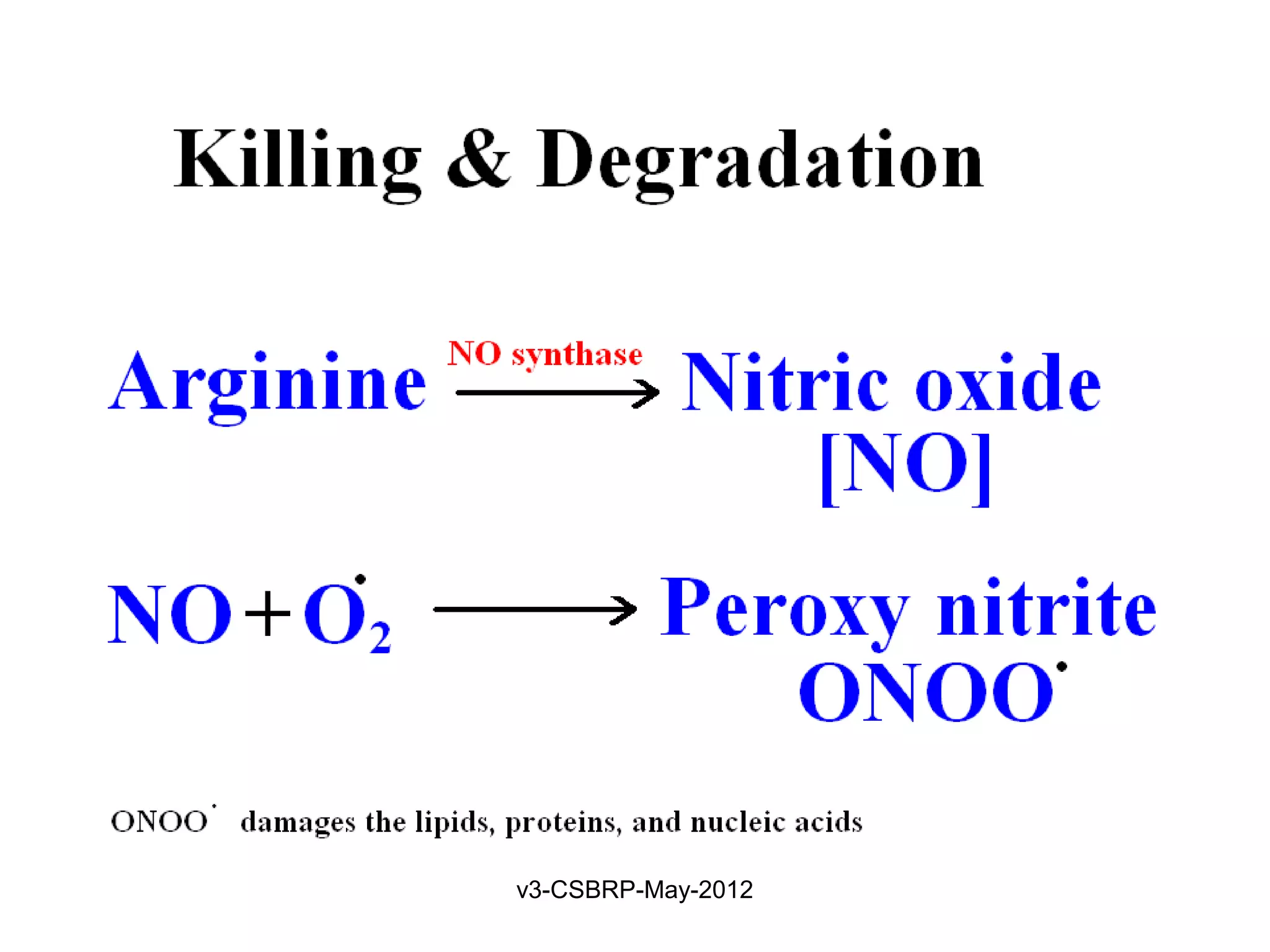
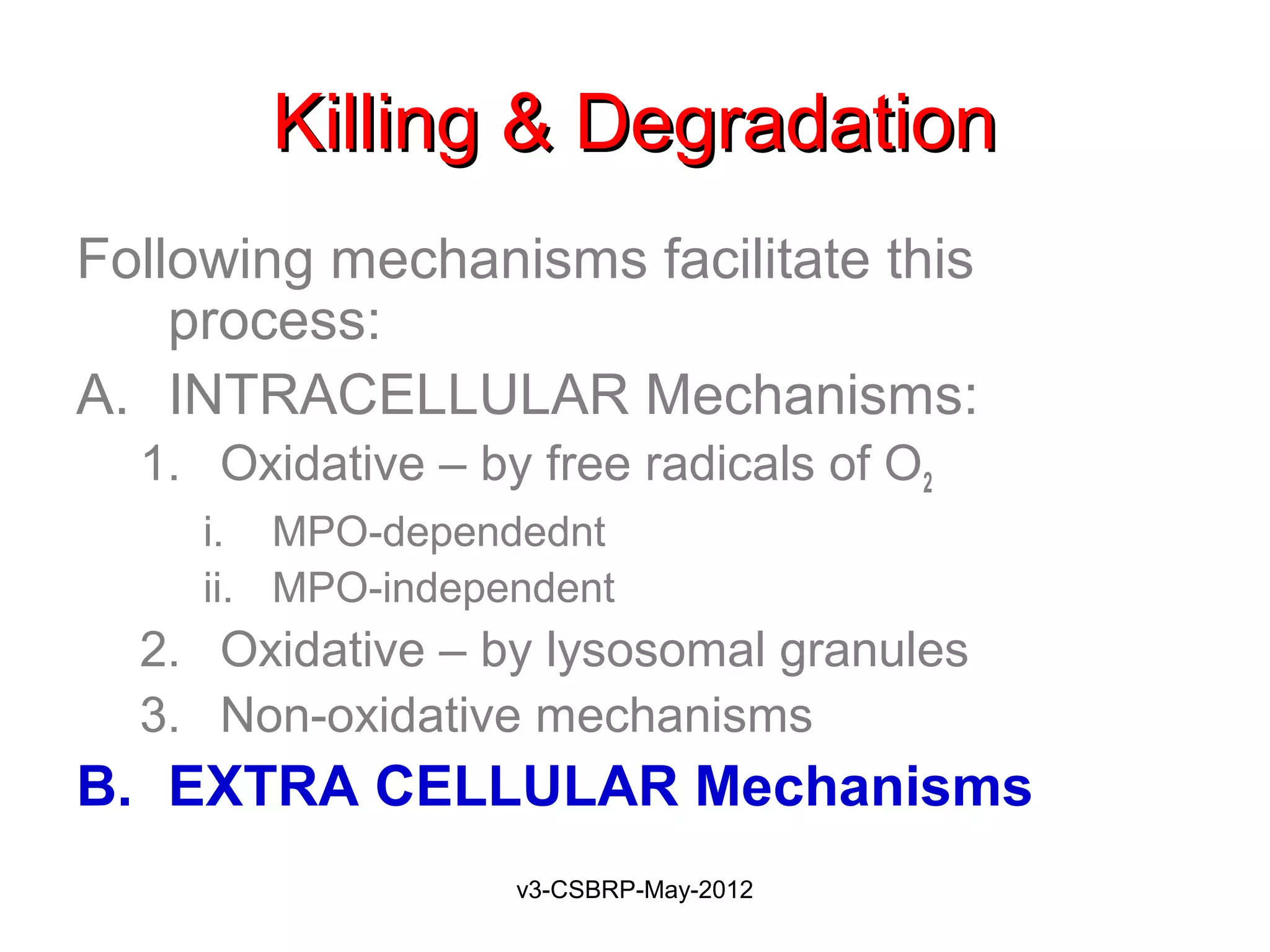
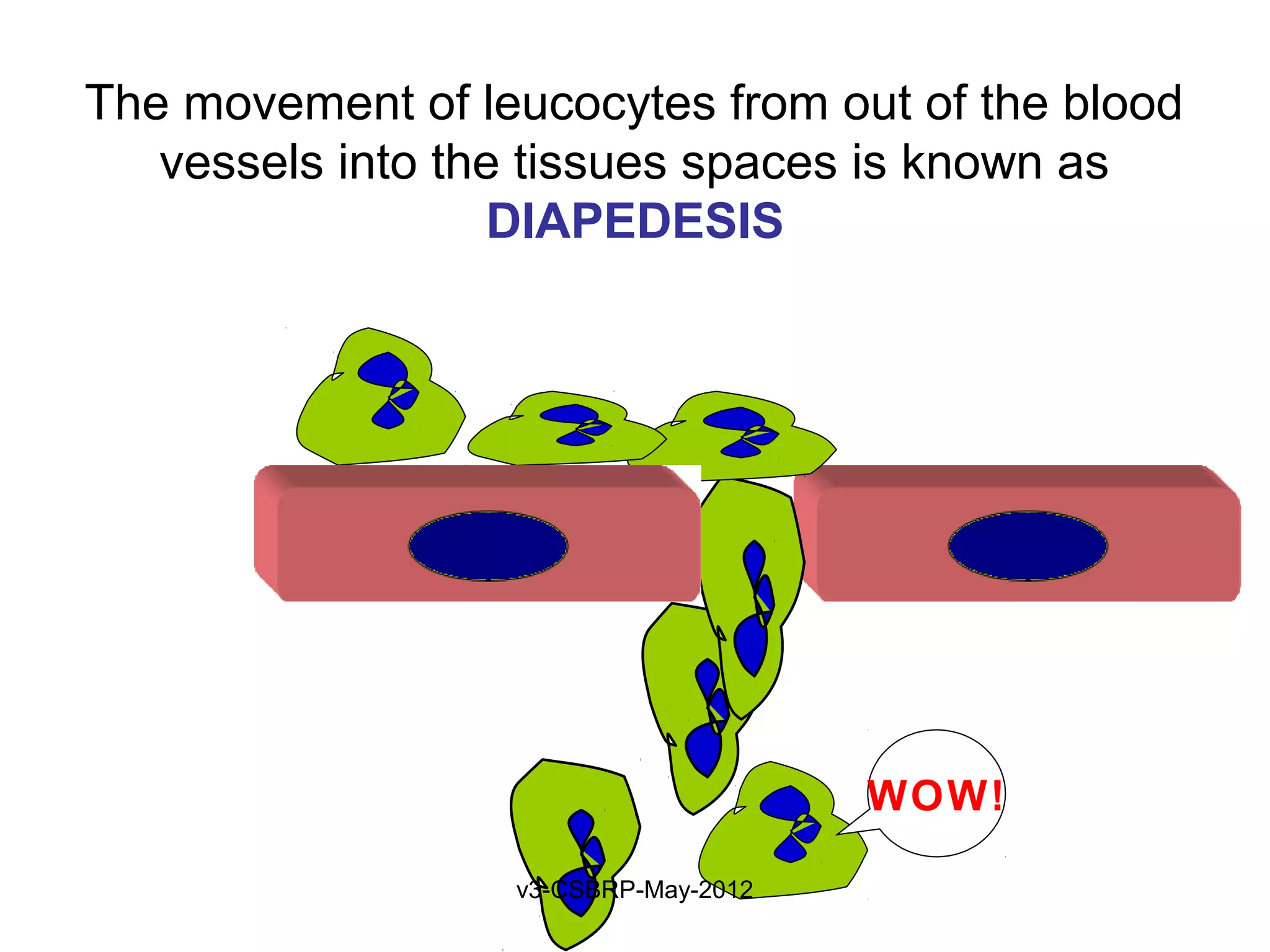
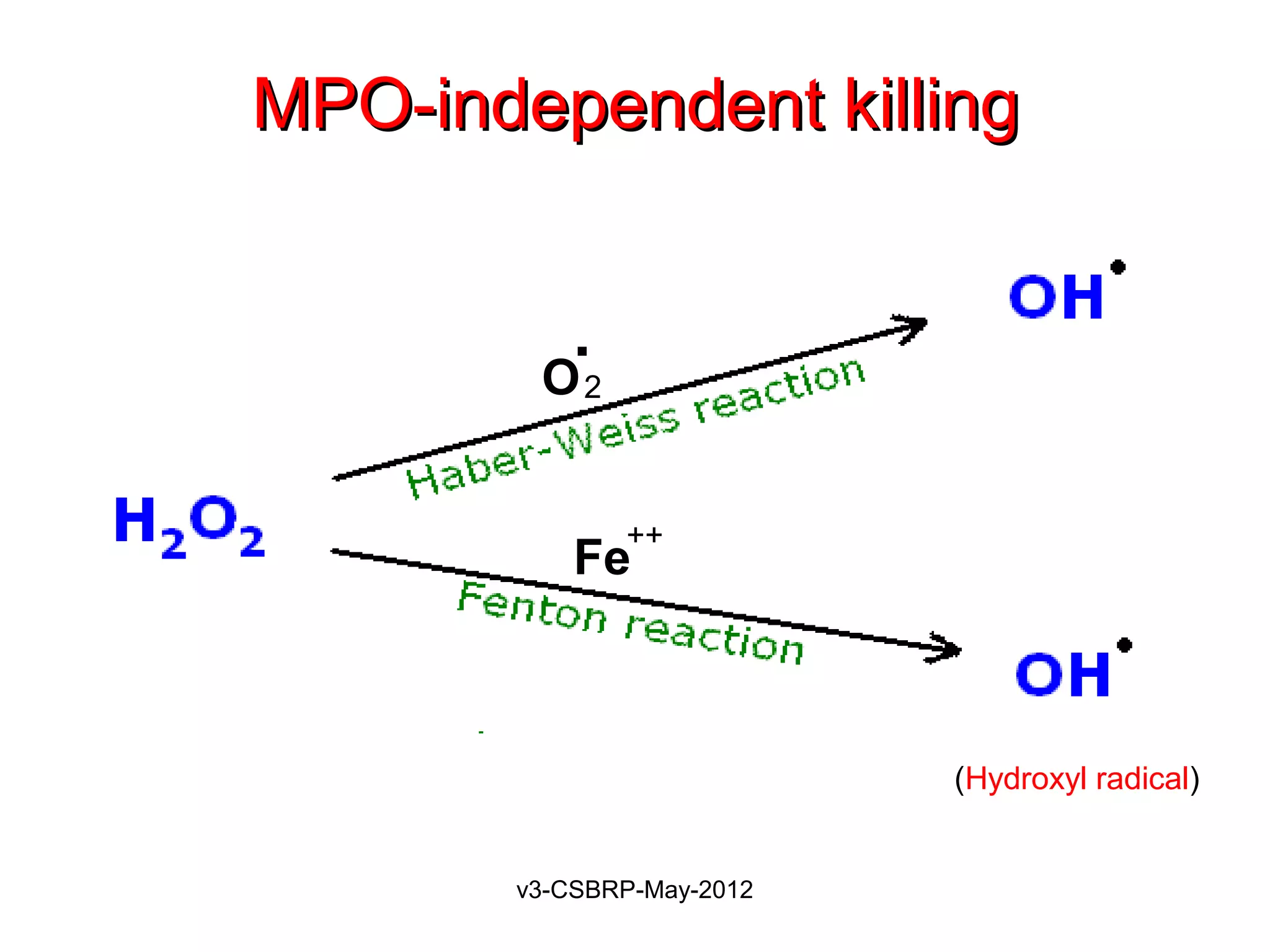
The document then discusses the removal of microbes via recognition by receptors, leukocyte activation, phagocytosis, and intracellular and extracellular killing mechanisms. It also notes

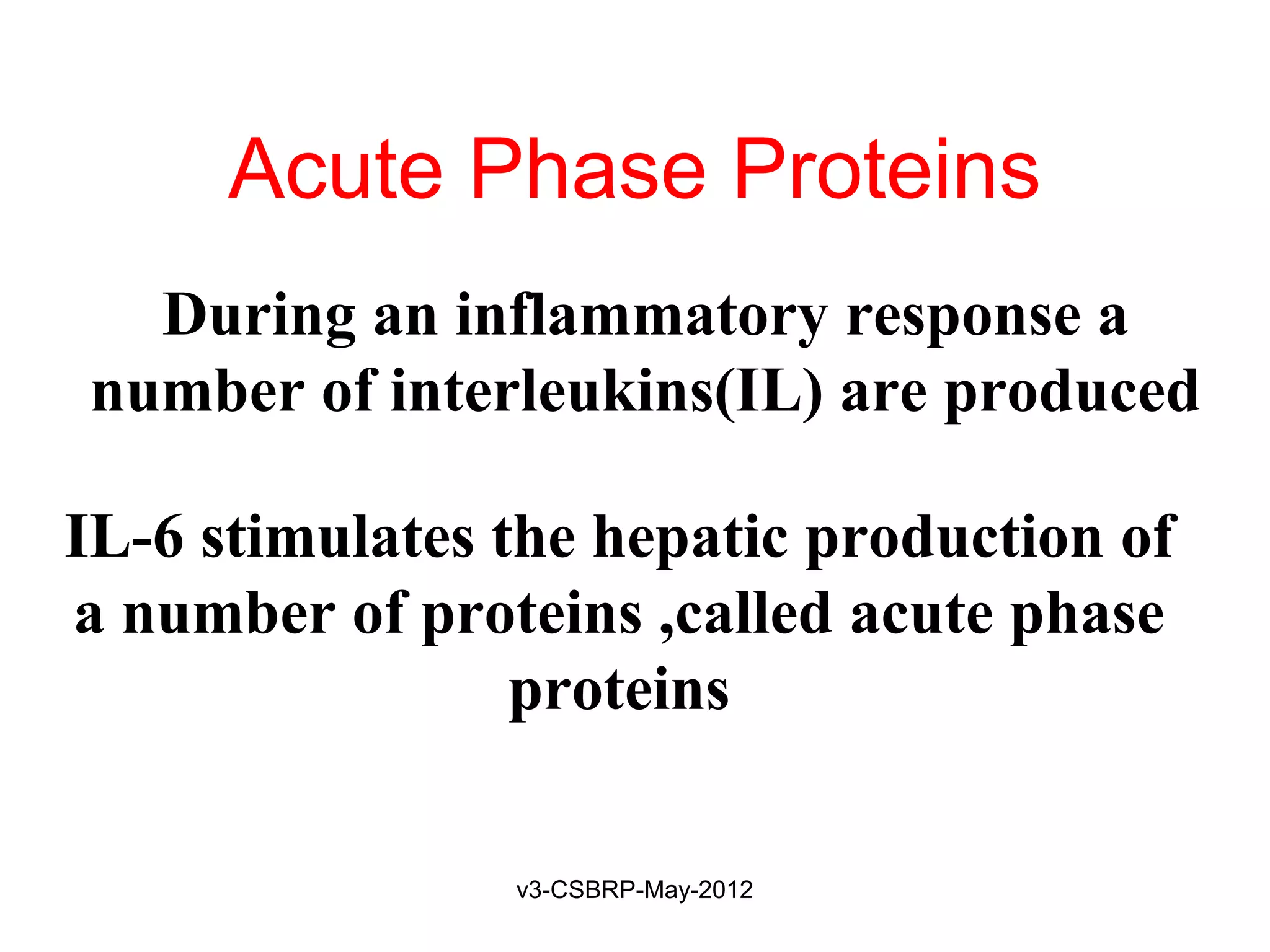
![Recognition of Microbes and Dead
Tissues
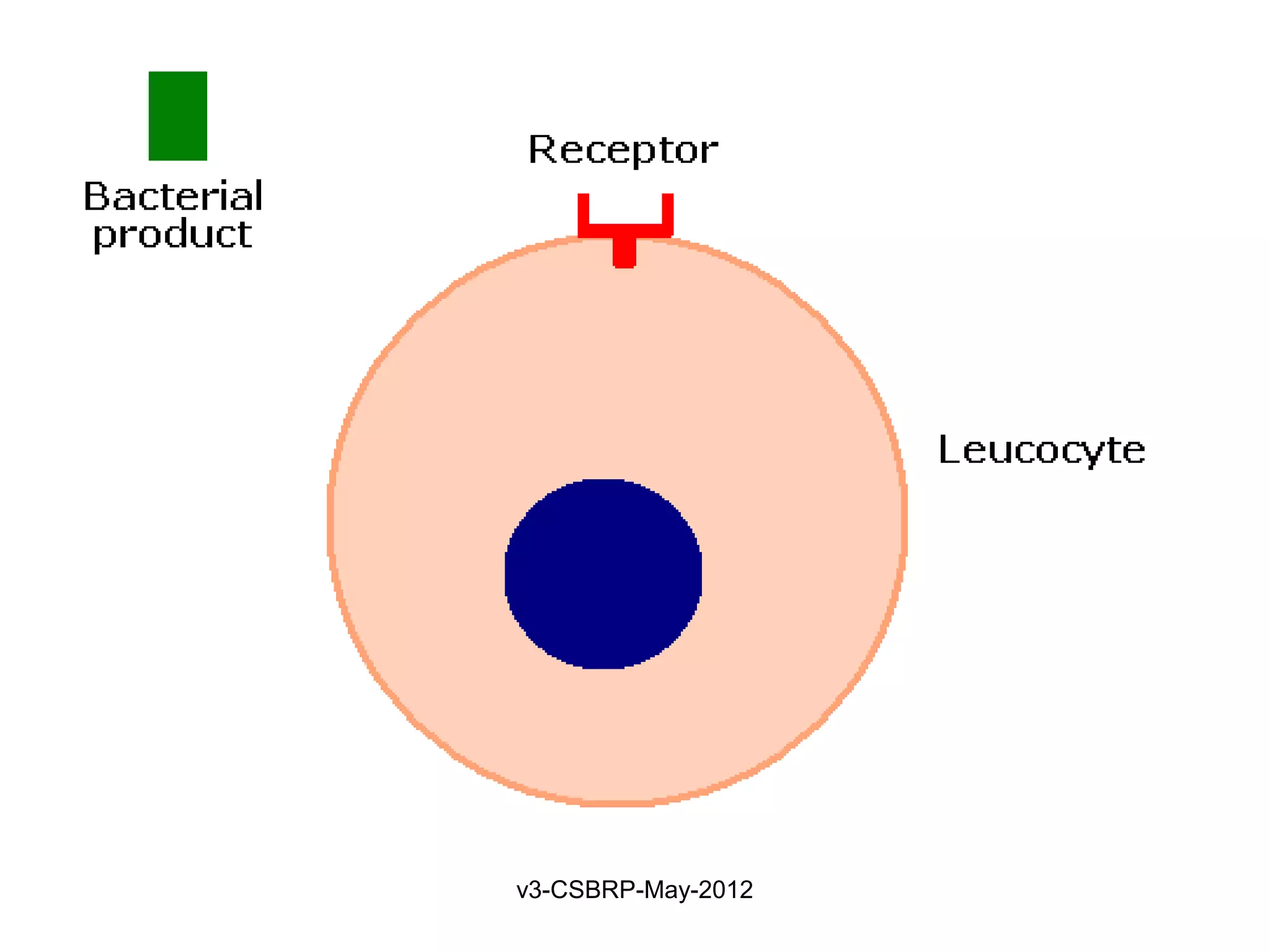
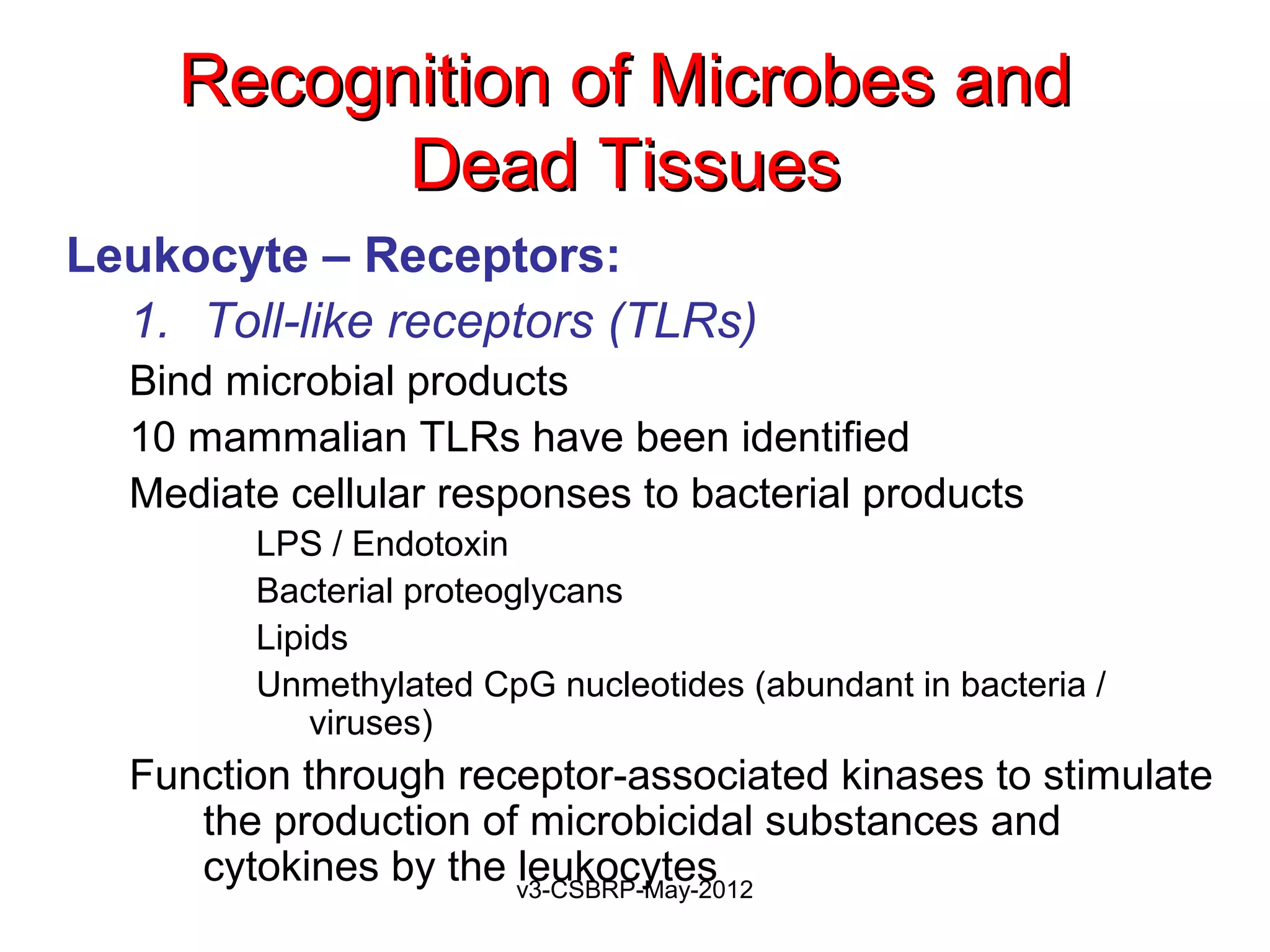
Leukocyte – Receptors:
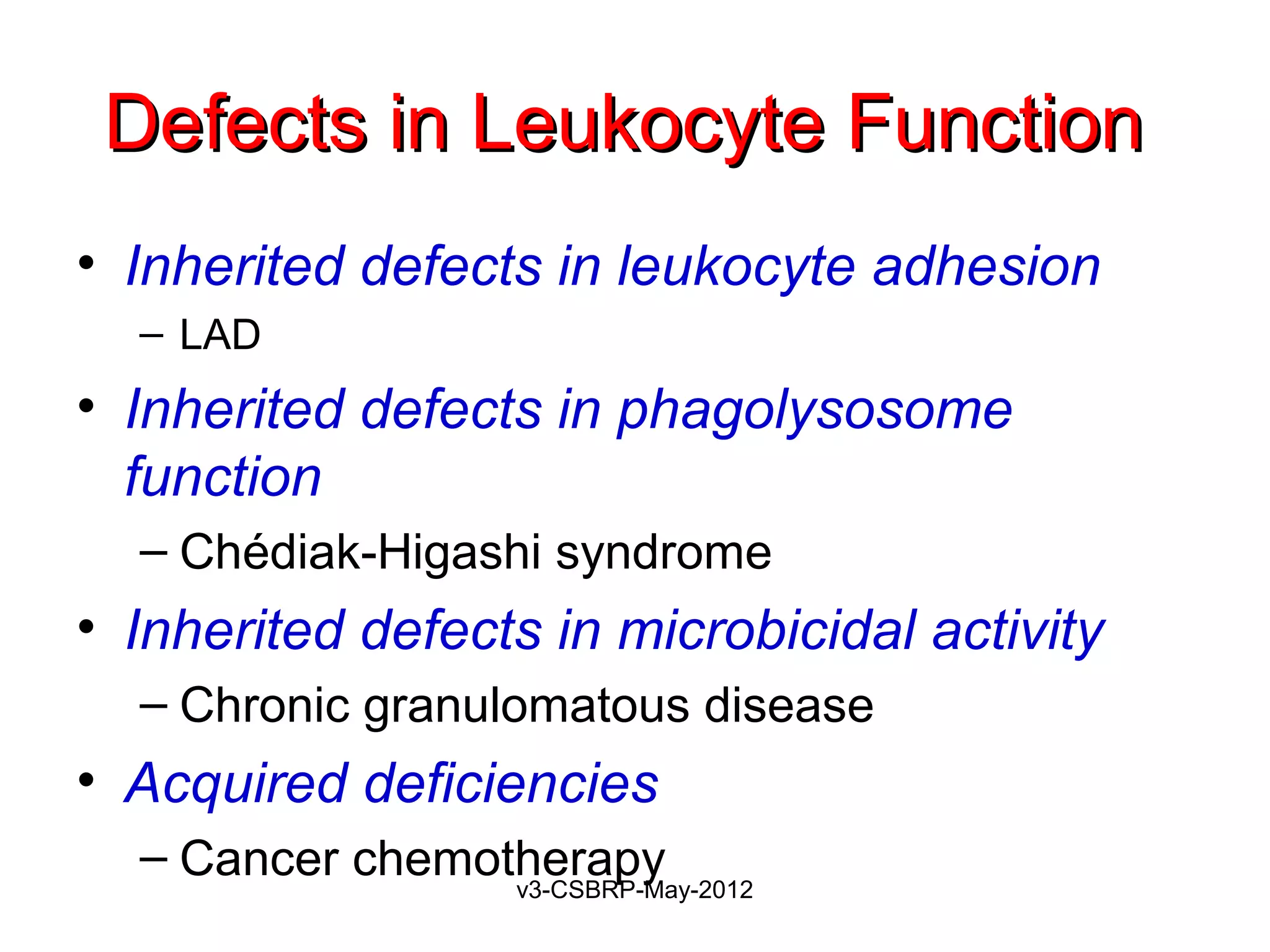
1. Toll-like receptors (TLRs)
2. G protein–coupled receptors
Found on neutrophils, macrophages
Recognize:
Bacterial peptides with N-formylmethionyl residues
Chemokines
Products of complement such as C5a
Lipid mediators [PAF, PGs, and LTs]
Ligand binding induces
Extravasation
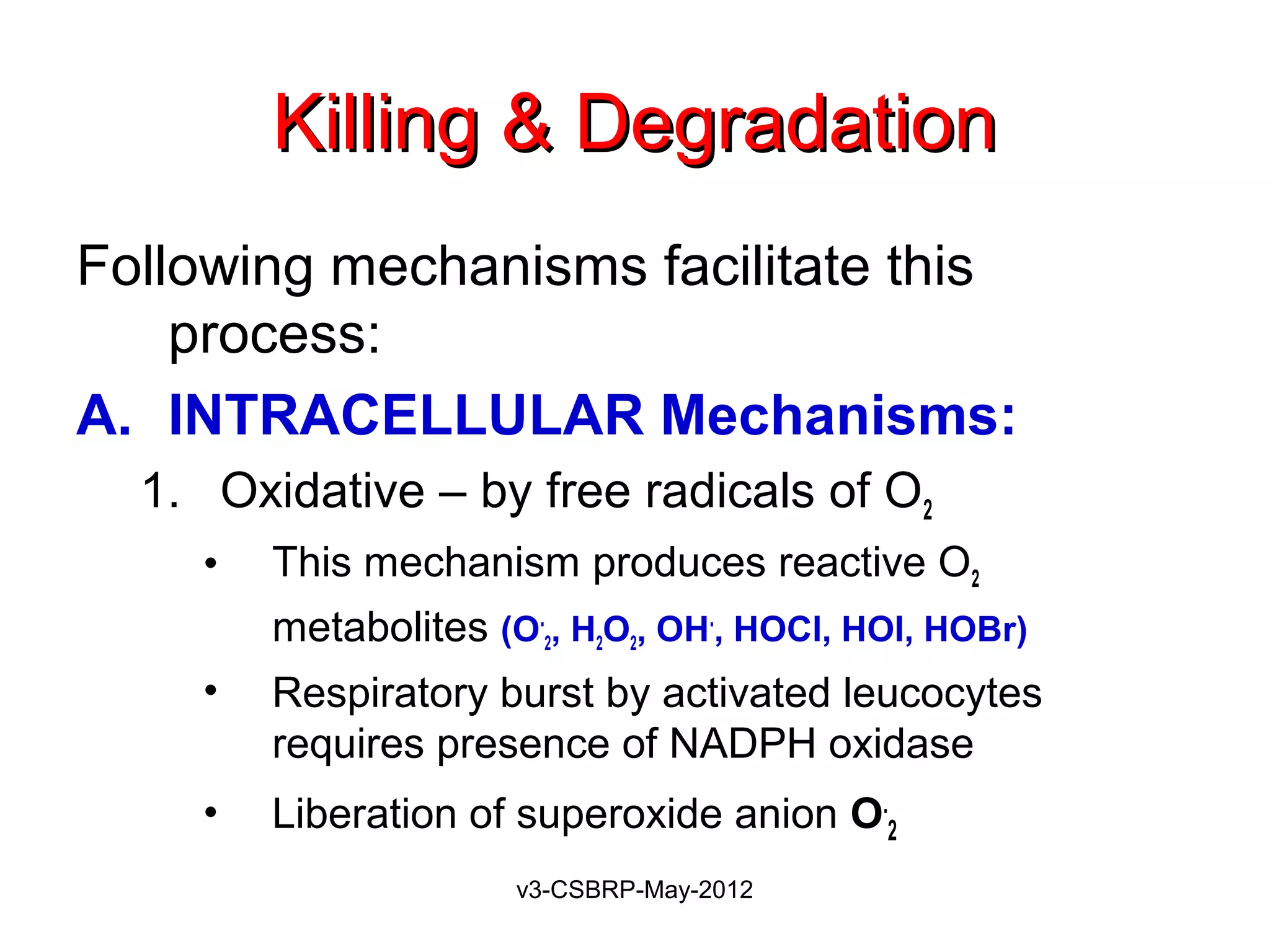
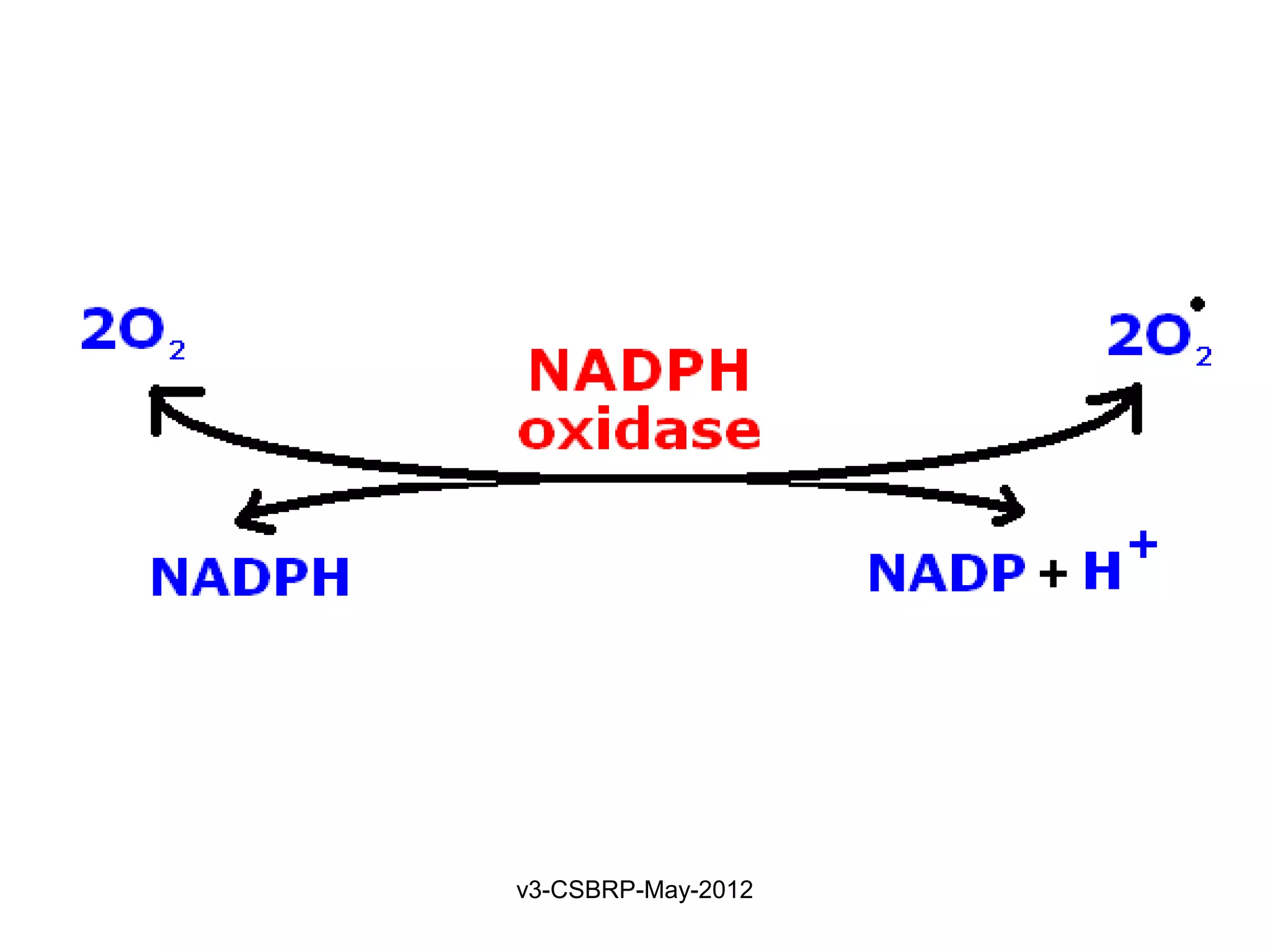
Production of microbicidal substances (ROS).
v3-CSBRP-May-2012](https://image.slidesharecdn.com/inflammation-4-csbrp-120815183156-phpapp02/75/Inflammation-4-9-2048.jpg)
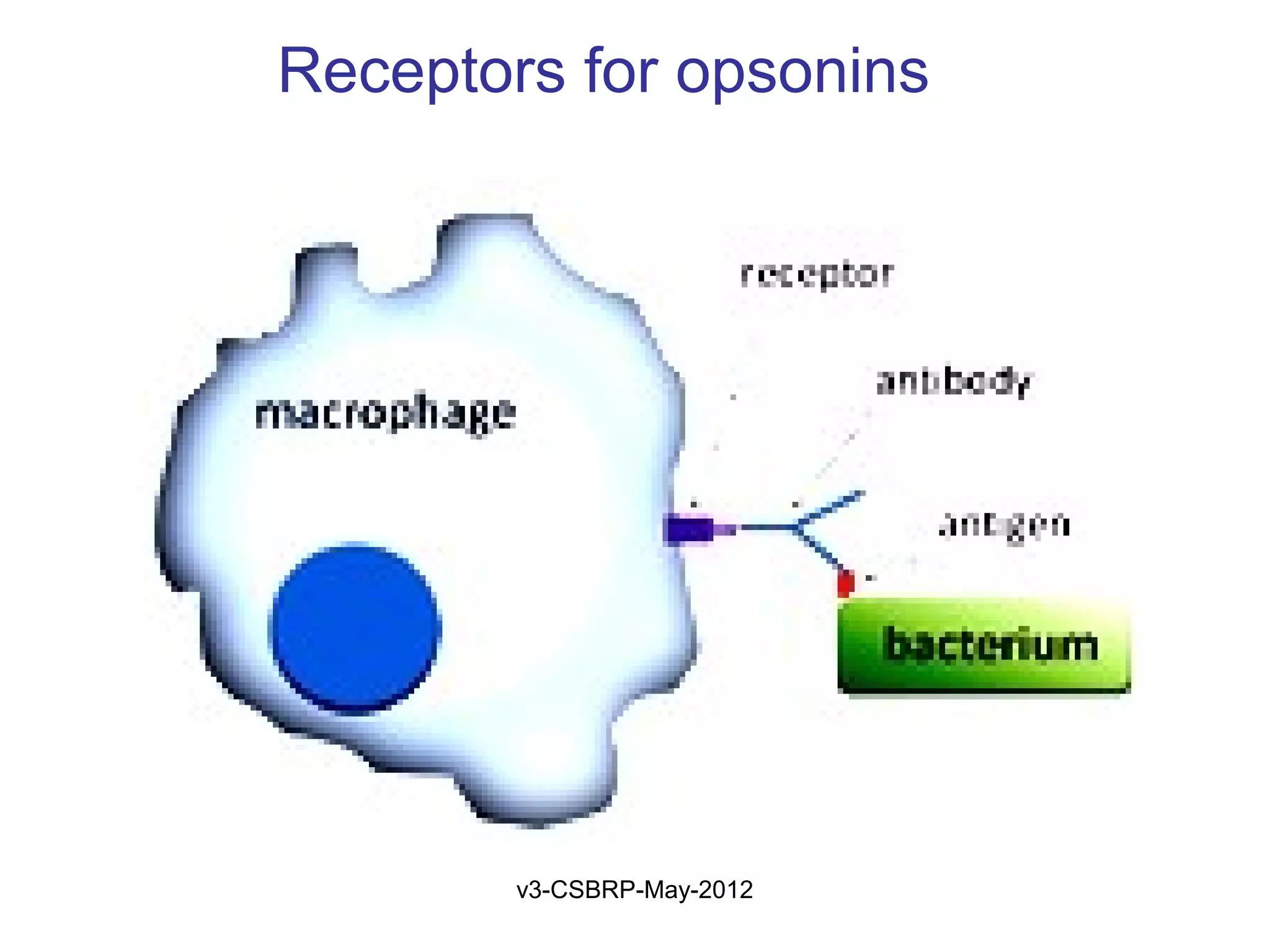
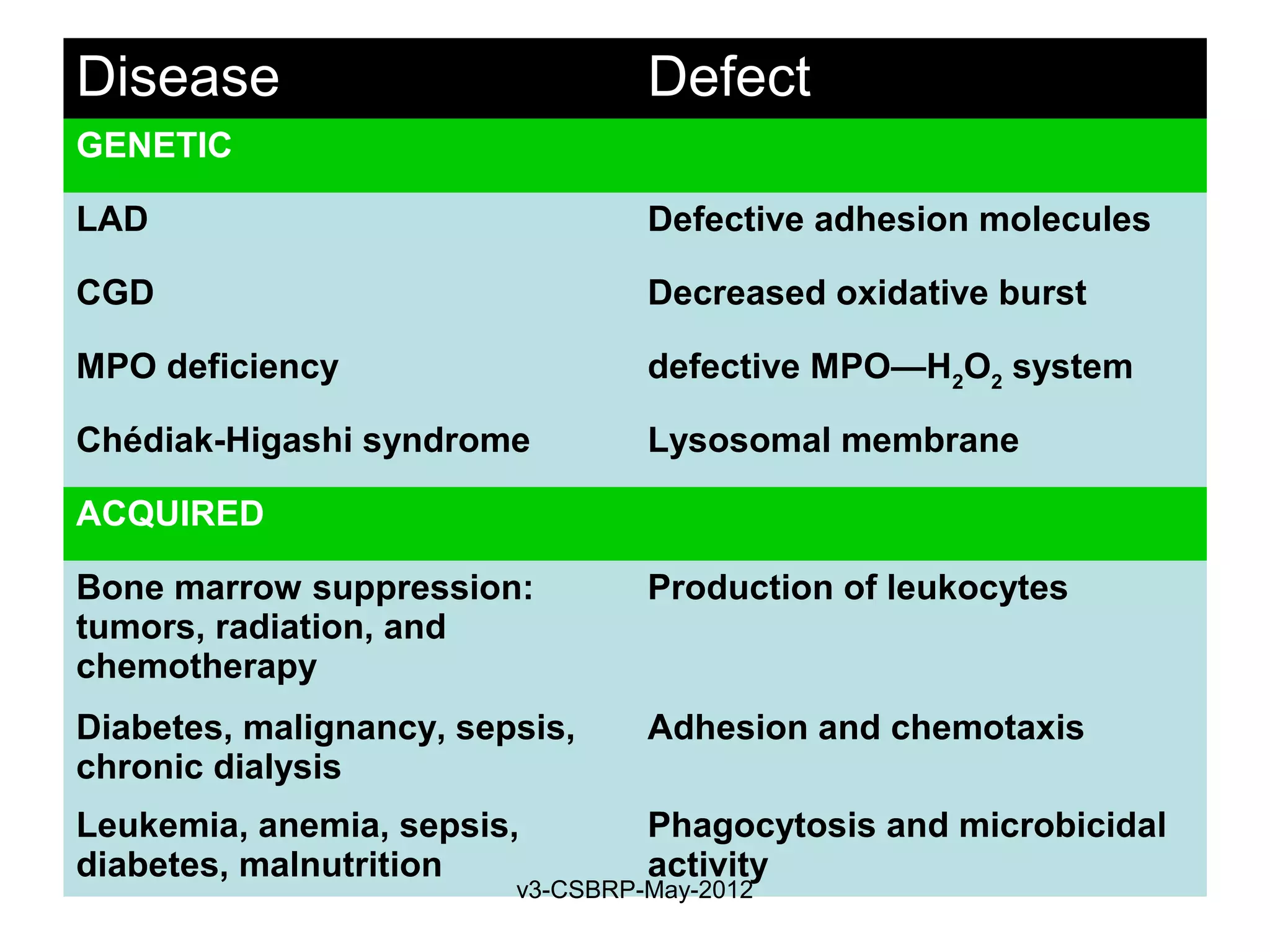
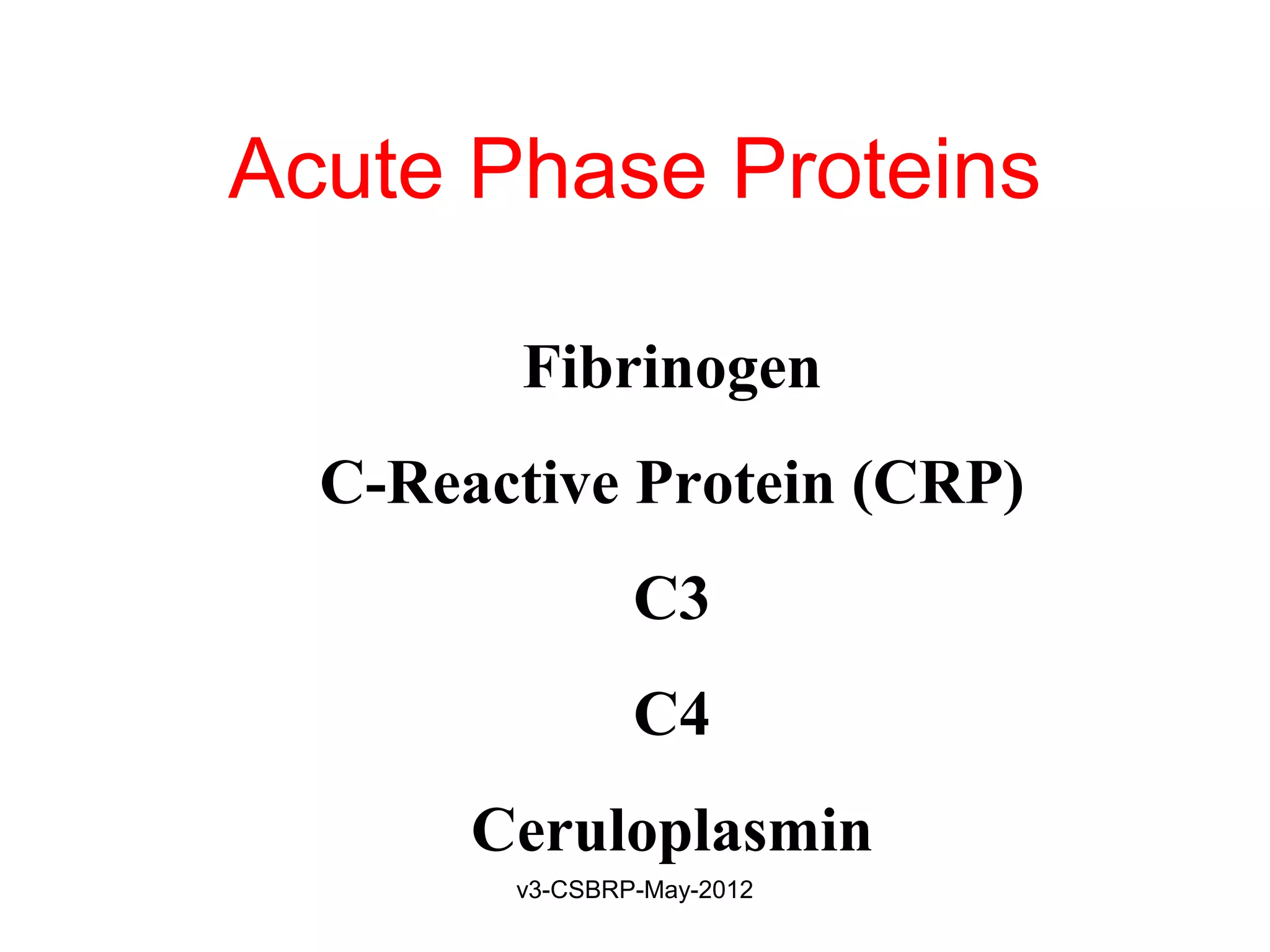
![Recognition of Microbes and
Dead Tissues
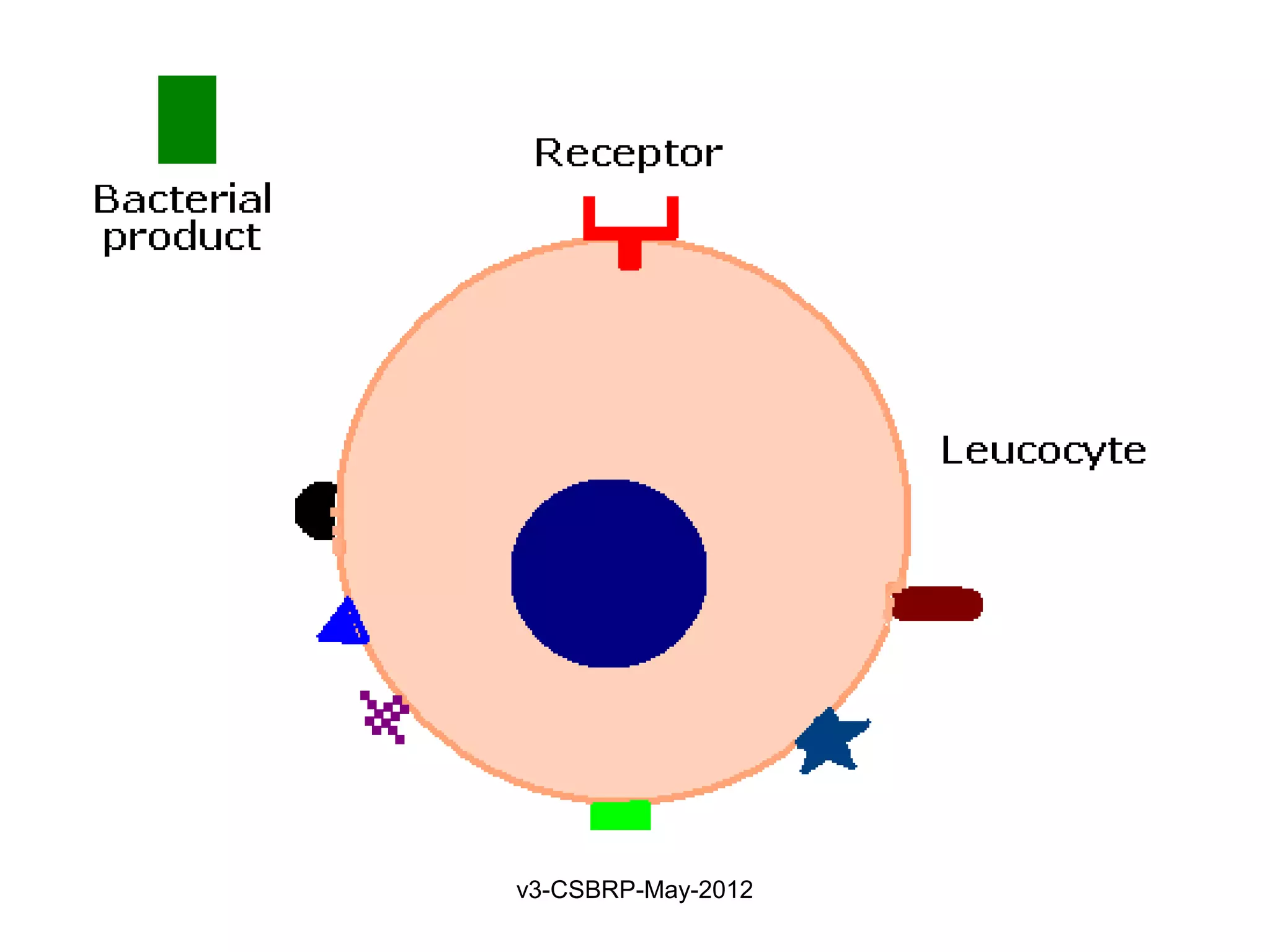
Leukocyte – Receptors:
1. Toll-like receptors (TLRs)
2. G protein–coupled receptors
3. Receptors for opsonins:
Leukocytes express receptors for proteins that coat
microbes
Opsonins include: Ig, C, and lectins
Phagocytes have:
• FcγRI (for Fc fragment)
• CR1 (for C3b)
• Receptor for plasma Lectins [mannan-binding lectin]
Receptors promotes phagocytosis
v3-CSBRP-May-2012](https://image.slidesharecdn.com/inflammation-4-csbrp-120815183156-phpapp02/75/Inflammation-4-10-2048.jpg)