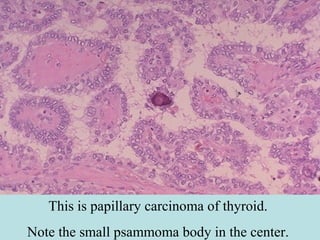
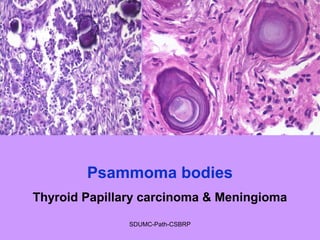
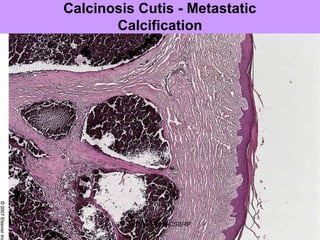
Pathologic calcification involves the abnormal deposition of calcium salts in tissues. There are two main types: dystrophic calcification occurs in dead or damaged tissues and is associated with normal calcium levels, while metastatic calcification occurs in normal living tissues and is associated with high calcium levels. Dystrophic calcification results from calcium deposition in areas of tissue damage or necrosis. Metastatic calcification is usually caused by hypercalcemia from conditions like hyperparathyroidism or certain cancers. The lungs, kidneys, and gastric mucosa are especially susceptible to metastatic calcification.


![Pathologic CalcificationPathologic Calcification
Pathologic calcification is the abnormal
tissue deposition of calcium salts
[together with smaller amounts of iron,
magnesium, and other mineral salts]
SDUMC-Path-CSBRP](https://image.slidesharecdn.com/cellinjuryadaptation-5-151101071849-lva1-app6891/85/Cell-injuryadaptation-5-3-320.jpg)
























![Pathology pearlsPathology pearls
• Calcification in lung fields usually
indicates benign process [Inflammatory
process]
• Calcification in breast usually indicates
malignant process [Carcinoma]
SDUMC-Path-CSBRP](https://image.slidesharecdn.com/cellinjuryadaptation-5-151101071849-lva1-app6891/85/Cell-injuryadaptation-5-31-320.jpg)


![• PTH acts directly on bone, where it
induces calcium resorption, and on the
kidney, where it stimulates calcium
reabsorption and synthesis of 1,25-
dihydroxyvitamin D [1,25(OH)2D], a
hormone that stimulates gastrointestinal
calcium absorption.
SDUMC-Path-CSBRP](https://image.slidesharecdn.com/cellinjuryadaptation-5-151101071849-lva1-app6891/85/Cell-injuryadaptation-5-34-320.jpg)




















