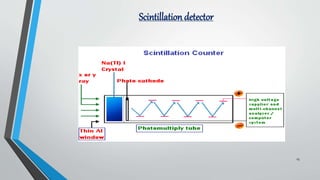
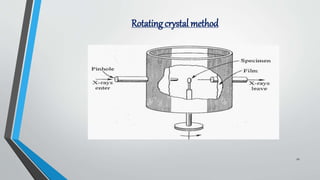
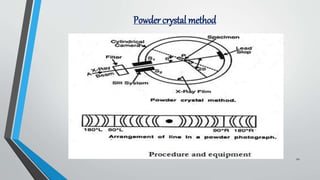
X-ray diffraction (XRD) is a crucial non-destructive analytical technique used for characterizing materials, identifying crystalline phases, and determining atomic structures. The document outlines the principles of XRD, its historical development, instrumentation, methods, applications, advantages, and limitations. It emphasizes XRD's significance in various fields, including materials science, engineering, and pharmaceuticals.