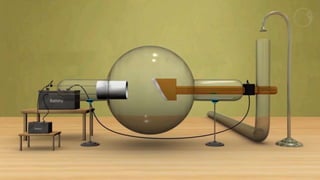
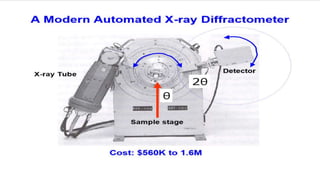
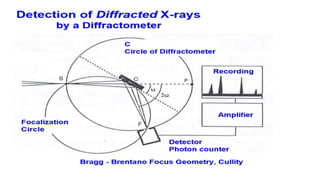
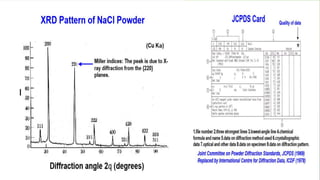
This document provides an overview of a student group presentation on the topic of X-ray diffraction. It includes an introduction to X-rays, the principle of X-ray diffraction, instrumentation used, methods, applications such as phase identification and determining crystal structure, and conclusions. The document also lists the group members and their roles and an outline of the content to be covered in the presentation.