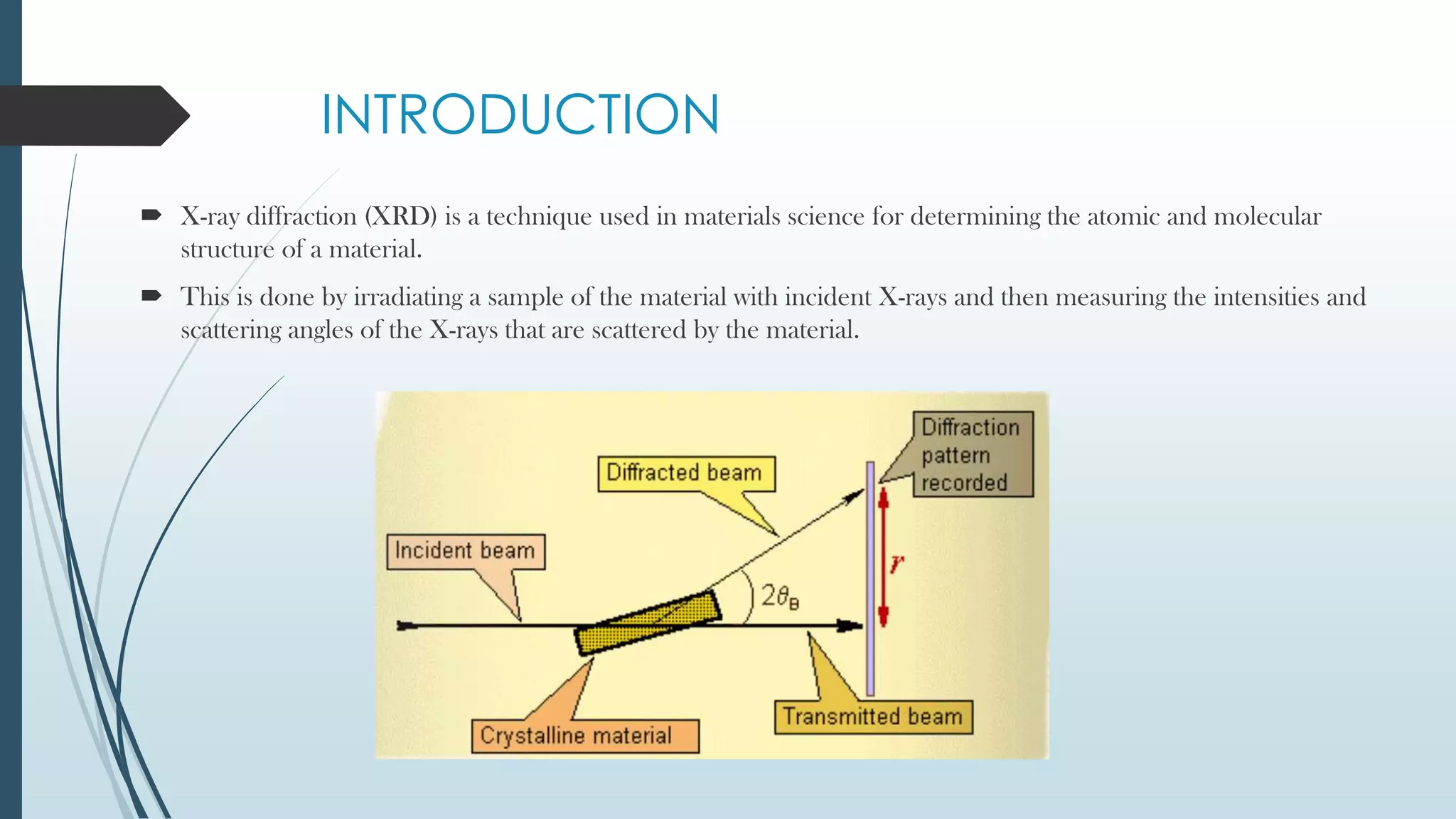
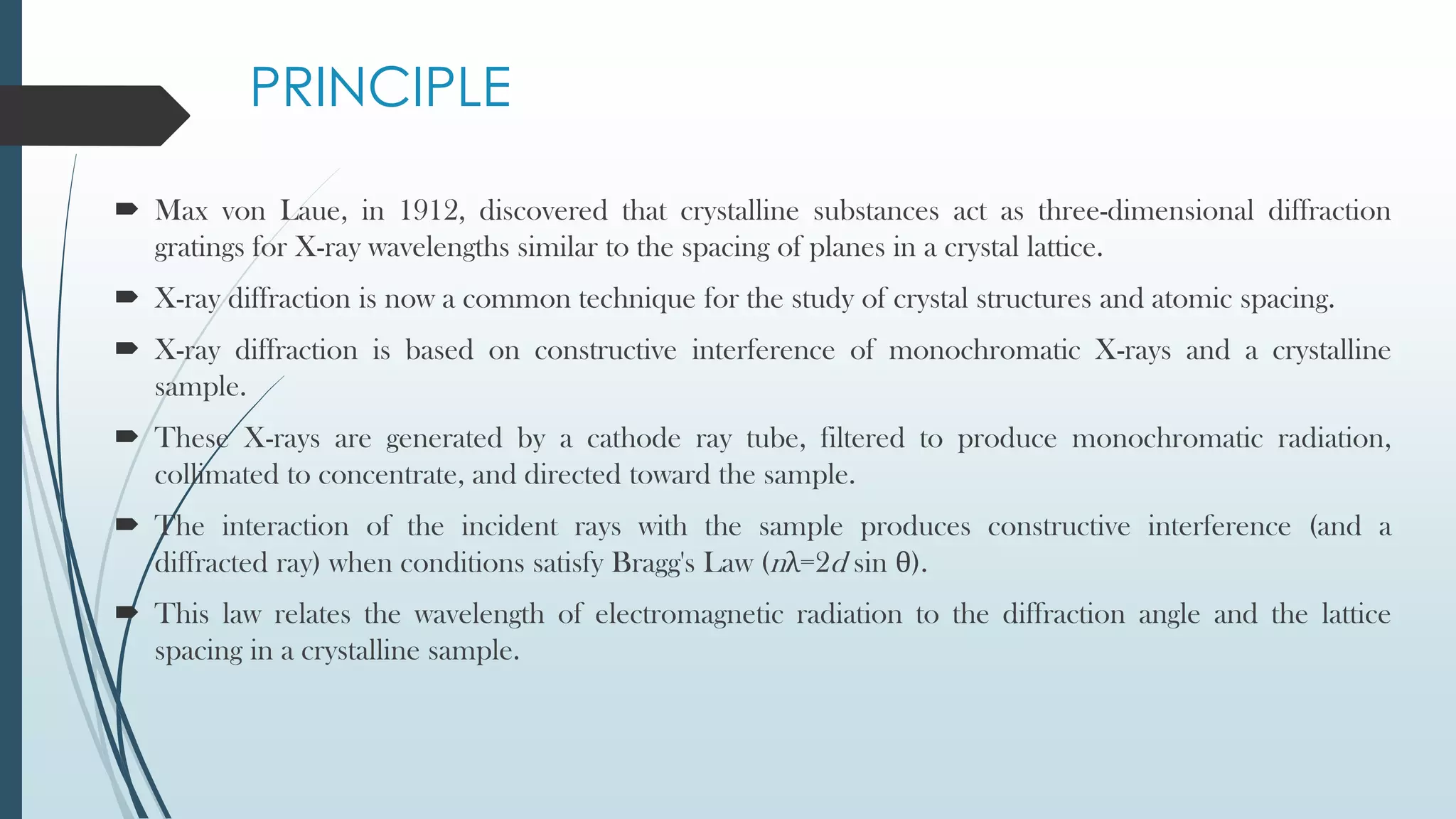
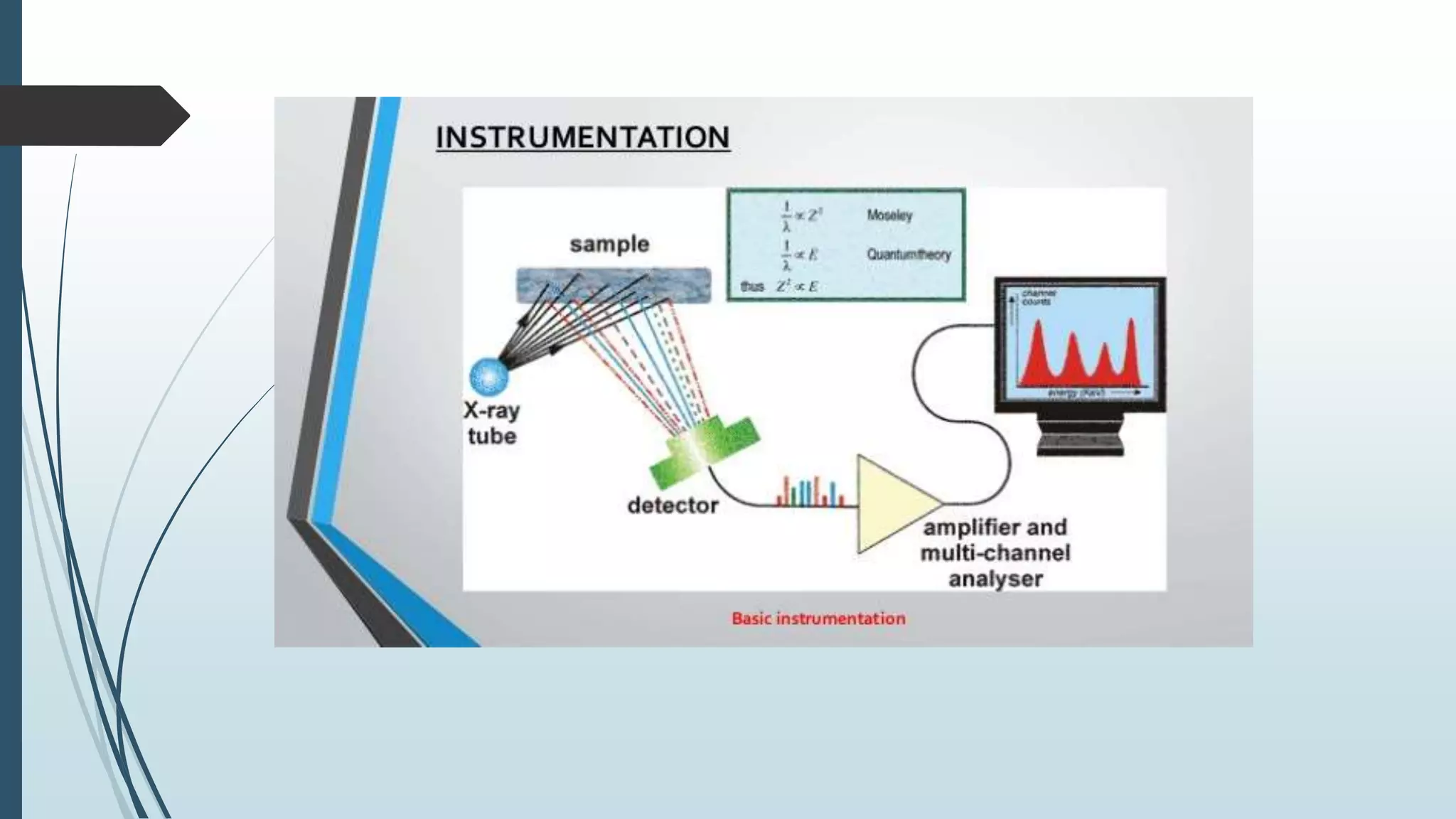
X-ray diffraction is a technique that uses X-rays to determine the atomic and molecular structure of crystals. When X-rays hit a crystal, they cause the atoms to diffract into specific patterns determined by Bragg's law. By analyzing these diffraction patterns, information about the crystal structure such as lattice parameters and spacing between atomic planes can be determined. Common applications of XRD include identifying materials and determining their purity, structure, and properties.






![XRD Benefits and Applications
XRD is a non-destructive technique used to [2]:
Identify crystalline phases and orientation
Determine structural properties:
- Lattice parameters
- Strain
- Grain size
- Epitaxy- type of crystal growth or material deposition in which new crystalline layers
- Phase composition
- Preferred orientation
Measure thickness of thin films and multi-layers
Determine atomic arrangement](https://image.slidesharecdn.com/x-raydiffraction-220106062323/75/X-ray-diffraction-12-2048.jpg)




