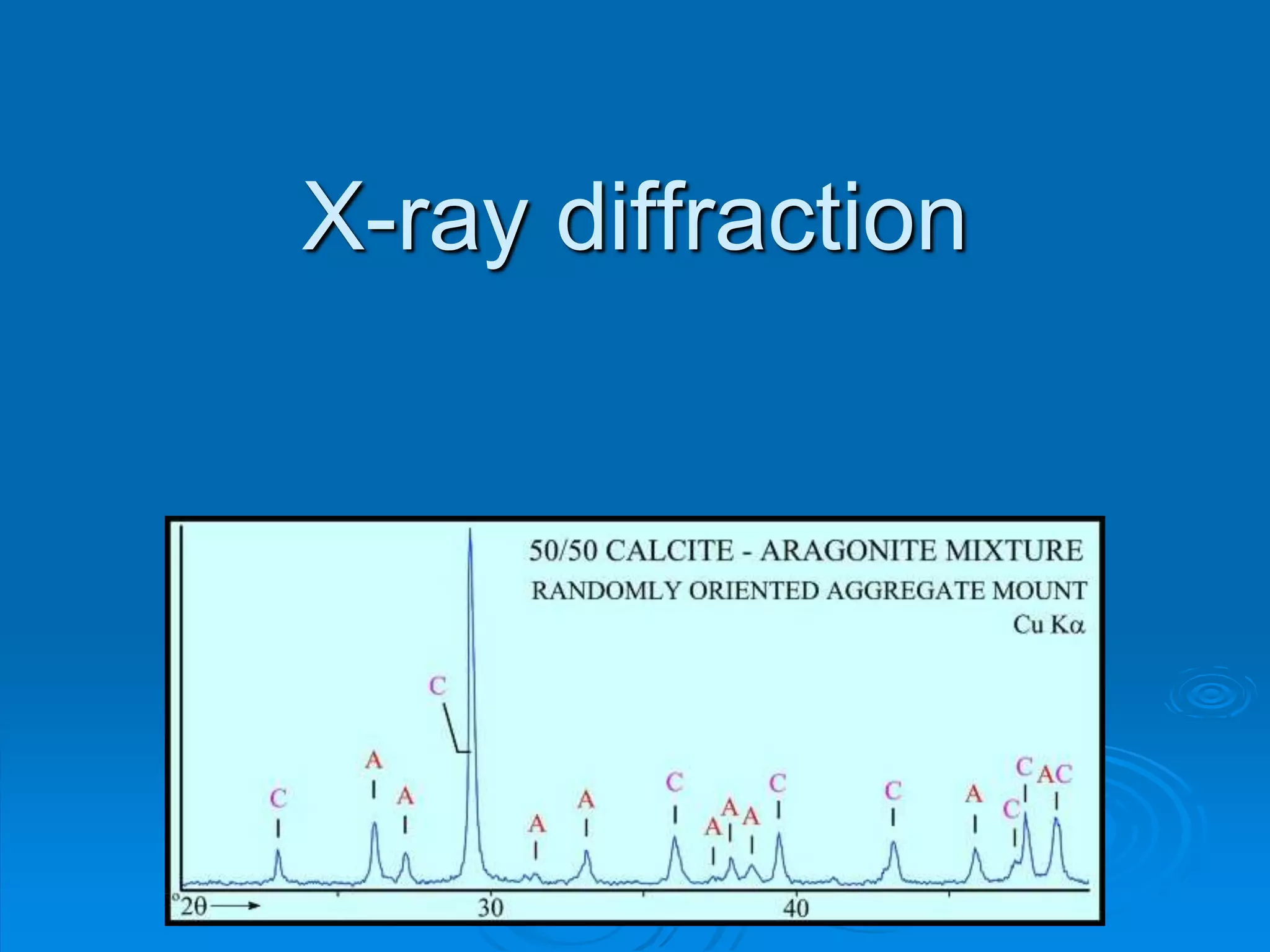
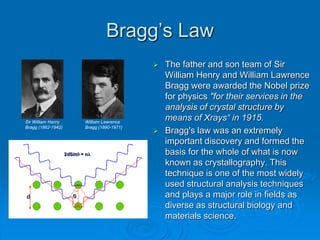
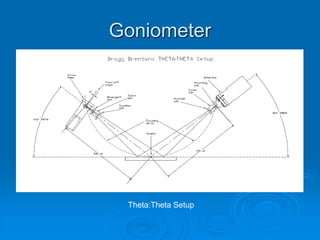
X-ray diffraction is a technique used to analyze the crystal structure of materials. Wilhelm Röntgen discovered X-rays in 1895, and Max von Laue discovered X-ray diffraction by crystals in 1912. Bragg's law, discovered in 1913, forms the basis for analyzing diffraction patterns to determine crystal structures. While traditionally useful, X-ray diffraction faces challenges in analyzing nanostructures due to their lack of long-range order and increased defects. Advances in detection technology and techniques have helped make X-ray diffraction applicable to characterizing nanomaterials.