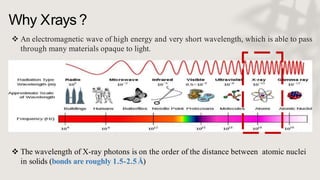
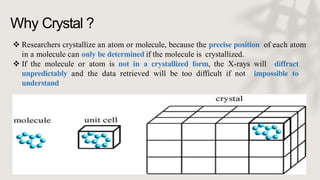
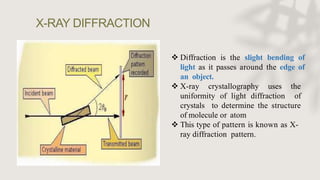
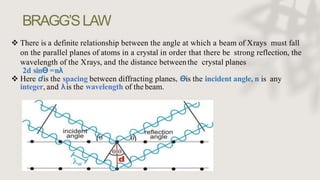
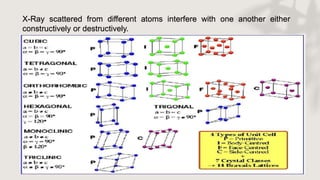
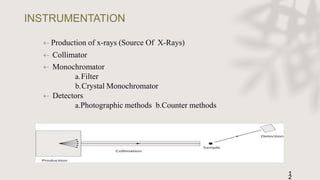
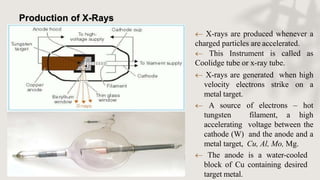
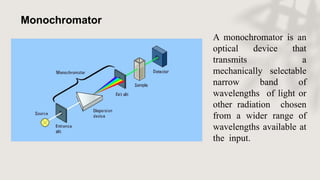
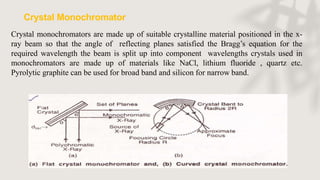
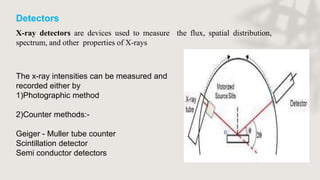
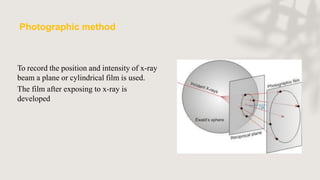
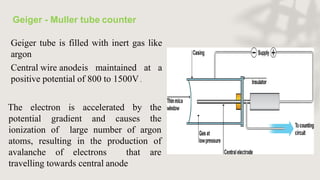
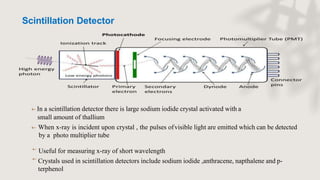
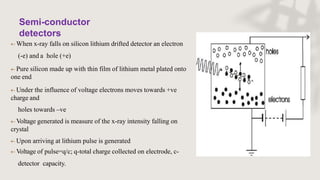
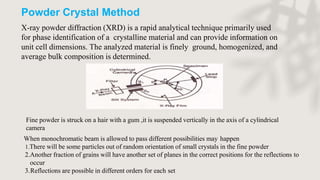
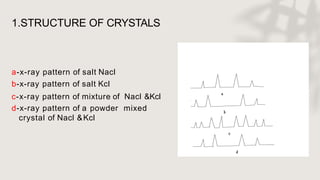
X-ray crystallography is a technique for determining the atomic and molecular structure of crystals by analyzing the diffraction patterns of X-rays as they interact with electrons in a crystallized molecule. The method relies on the precision of crystal formation to provide clear data, and uses Bragg's law to establish a relationship between the diffraction angle, wavelength, and distance between crystal planes. Various detection methods, including photographic and counter methods, are employed to measure the properties of X-rays and analyze crystallized structures.