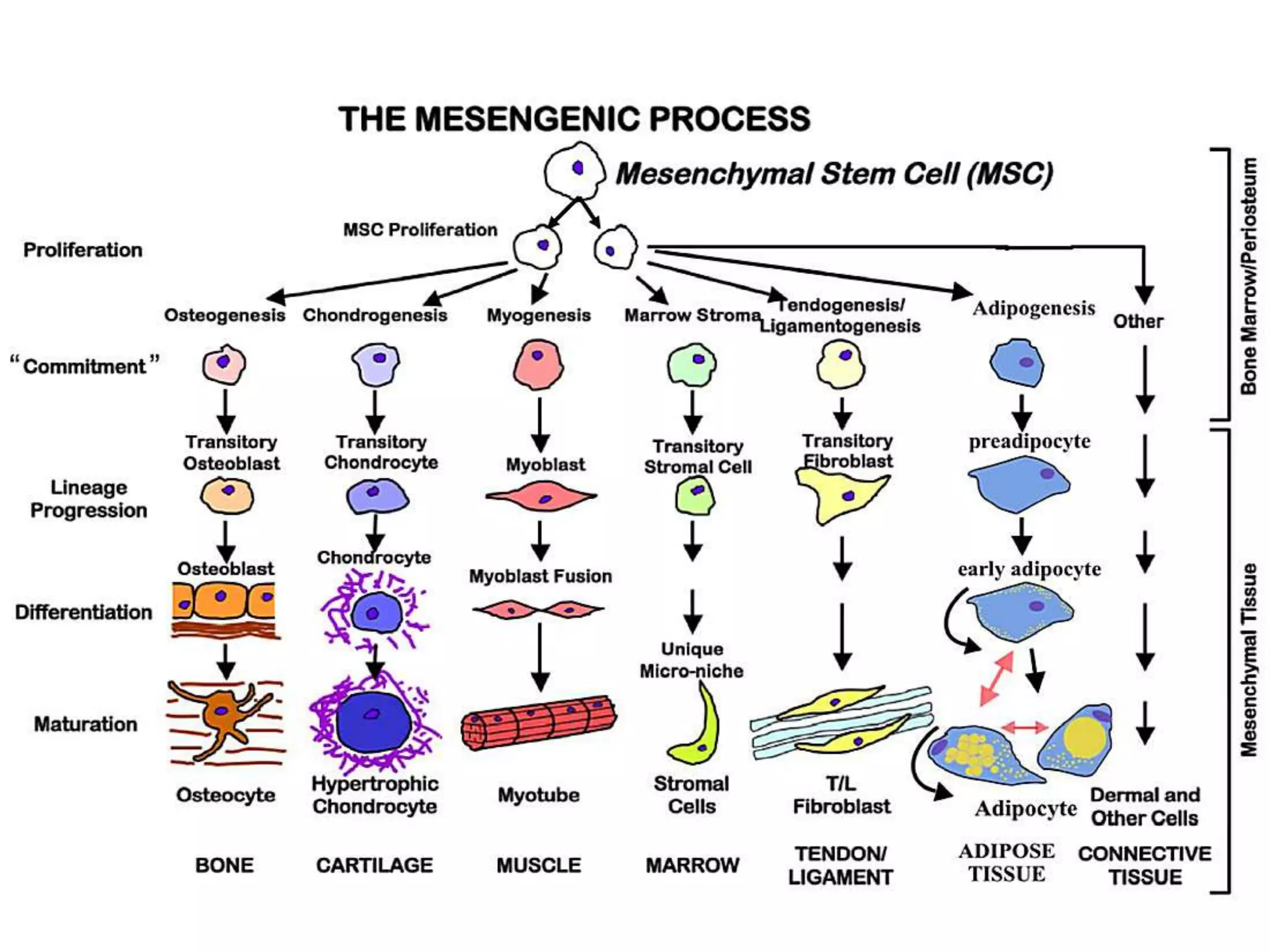
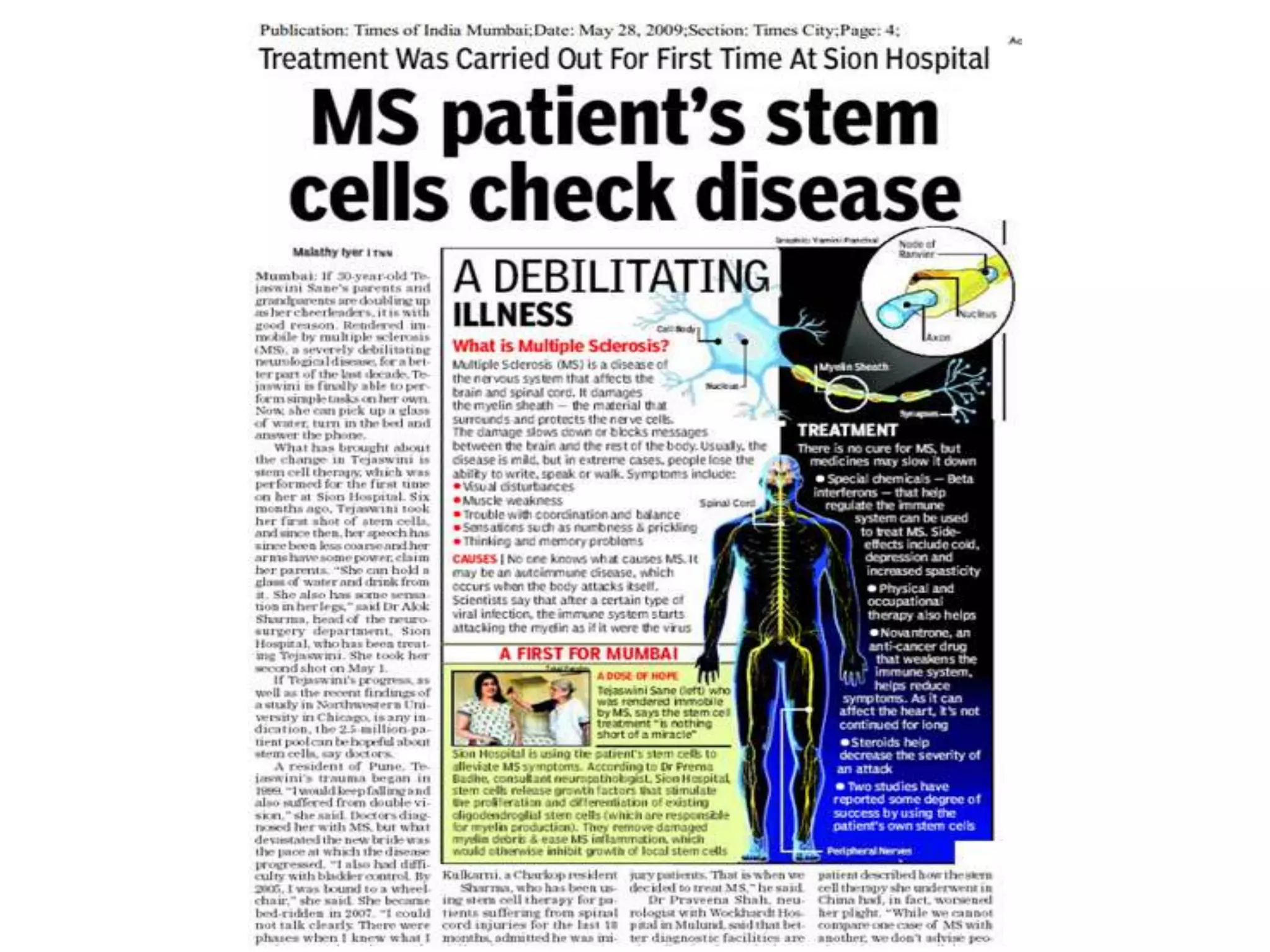
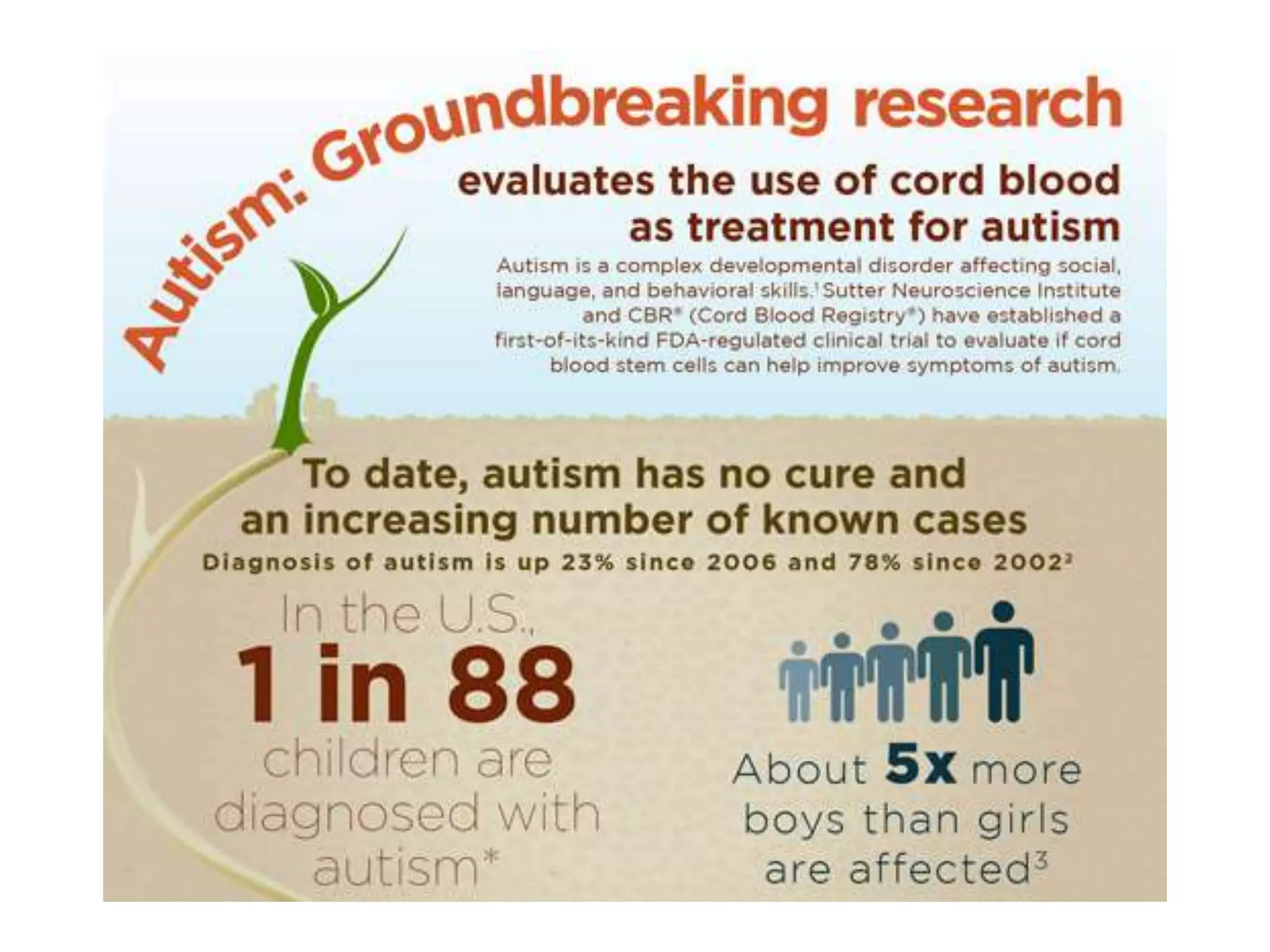
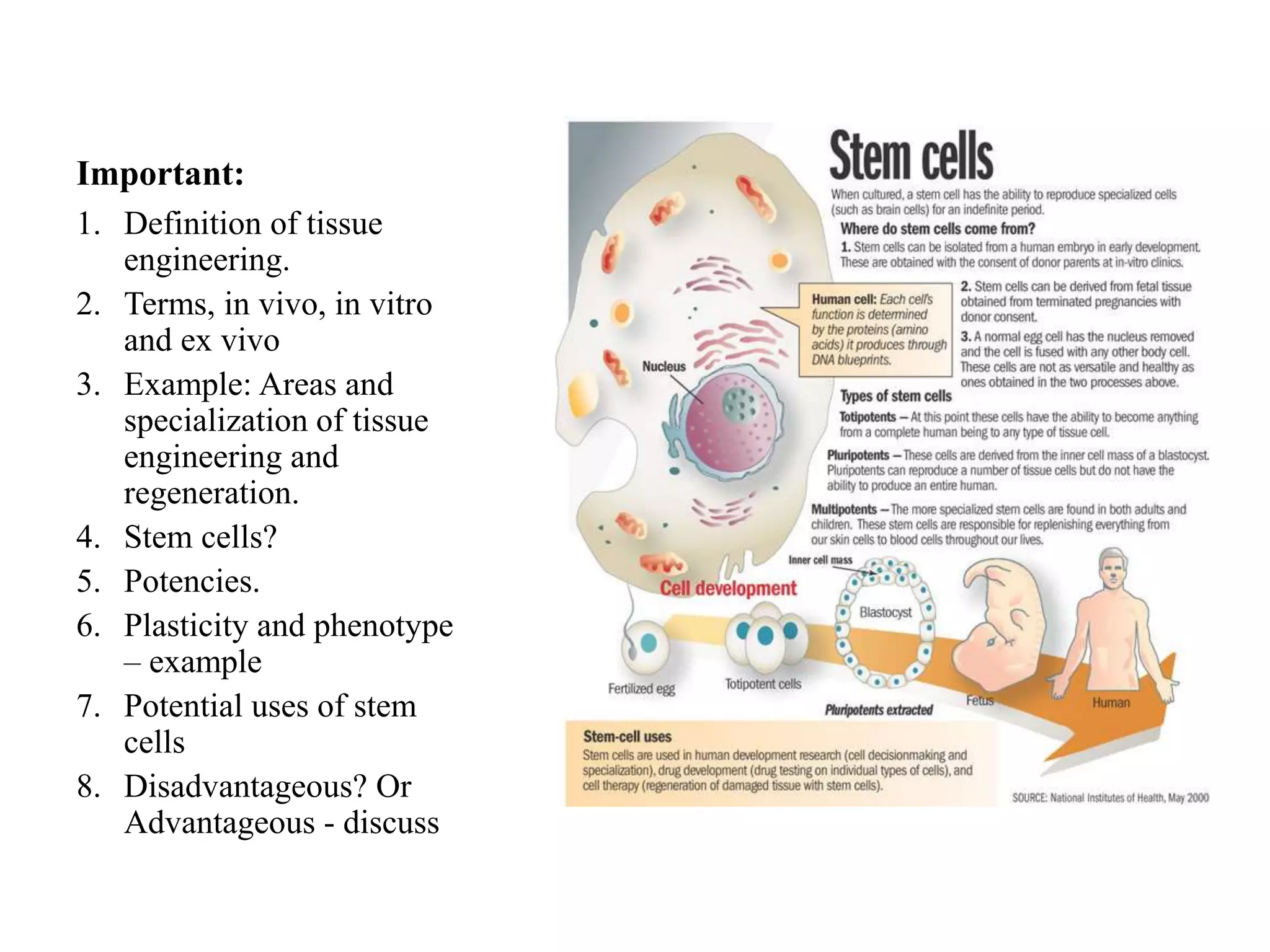
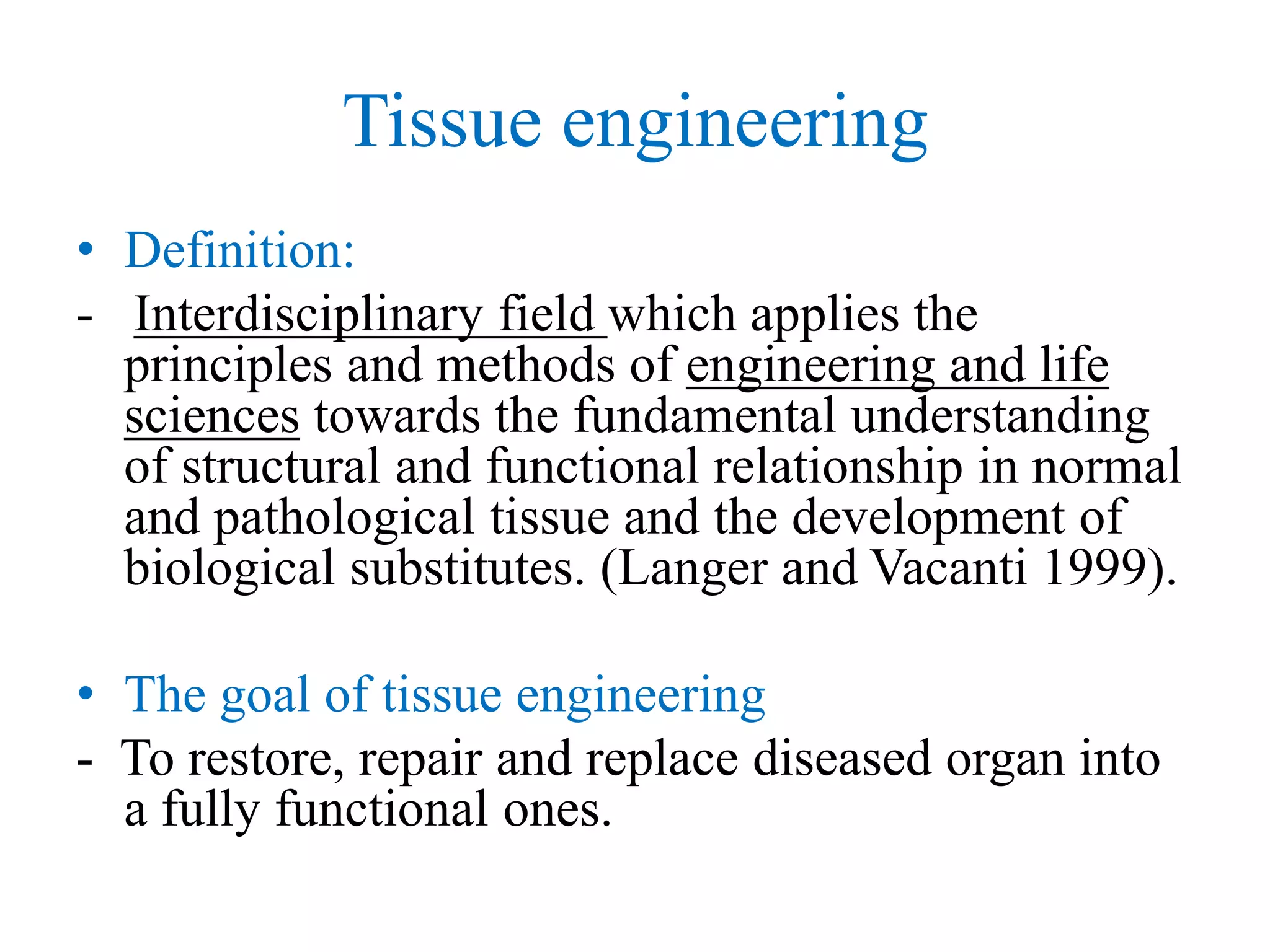
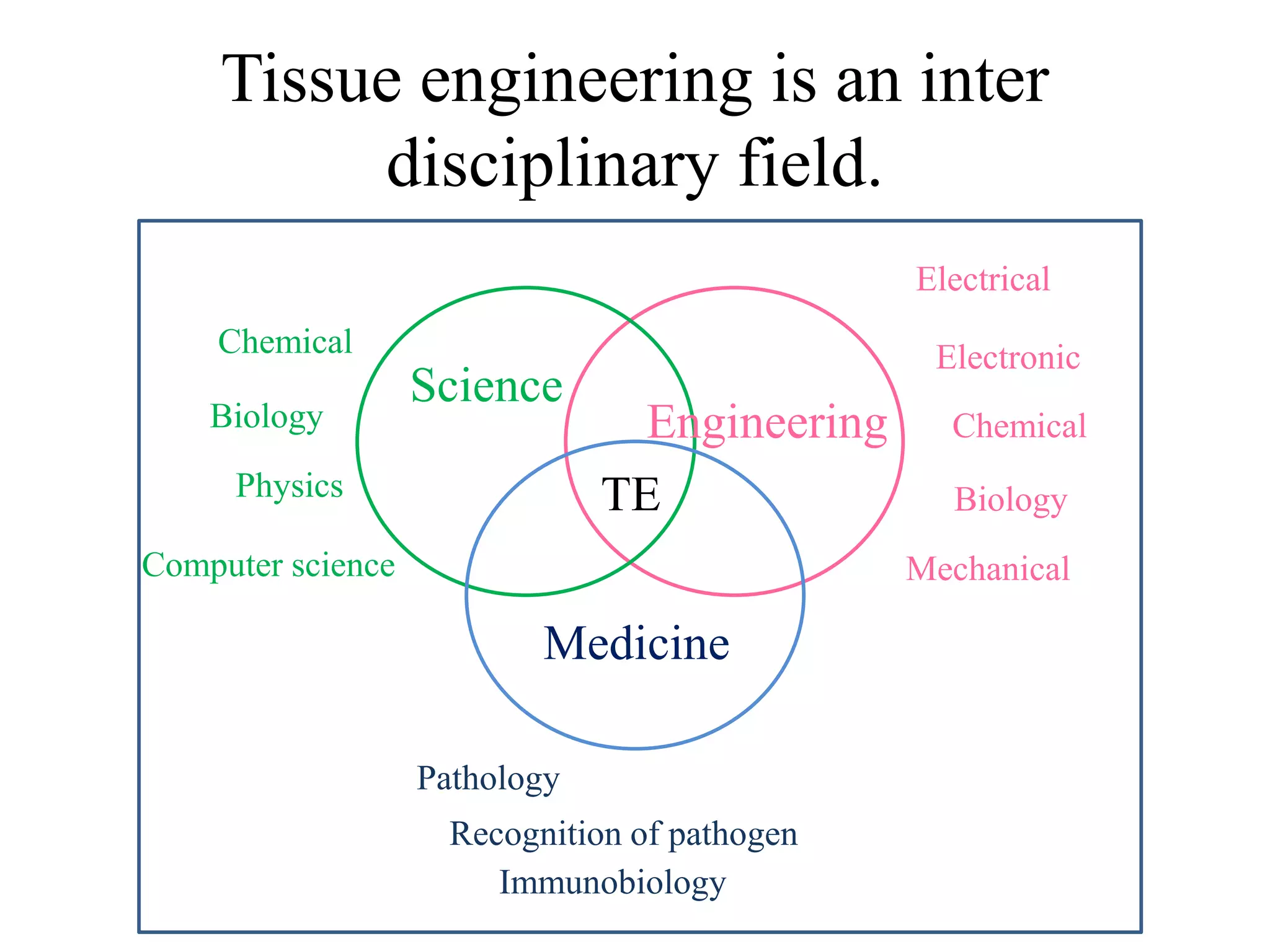
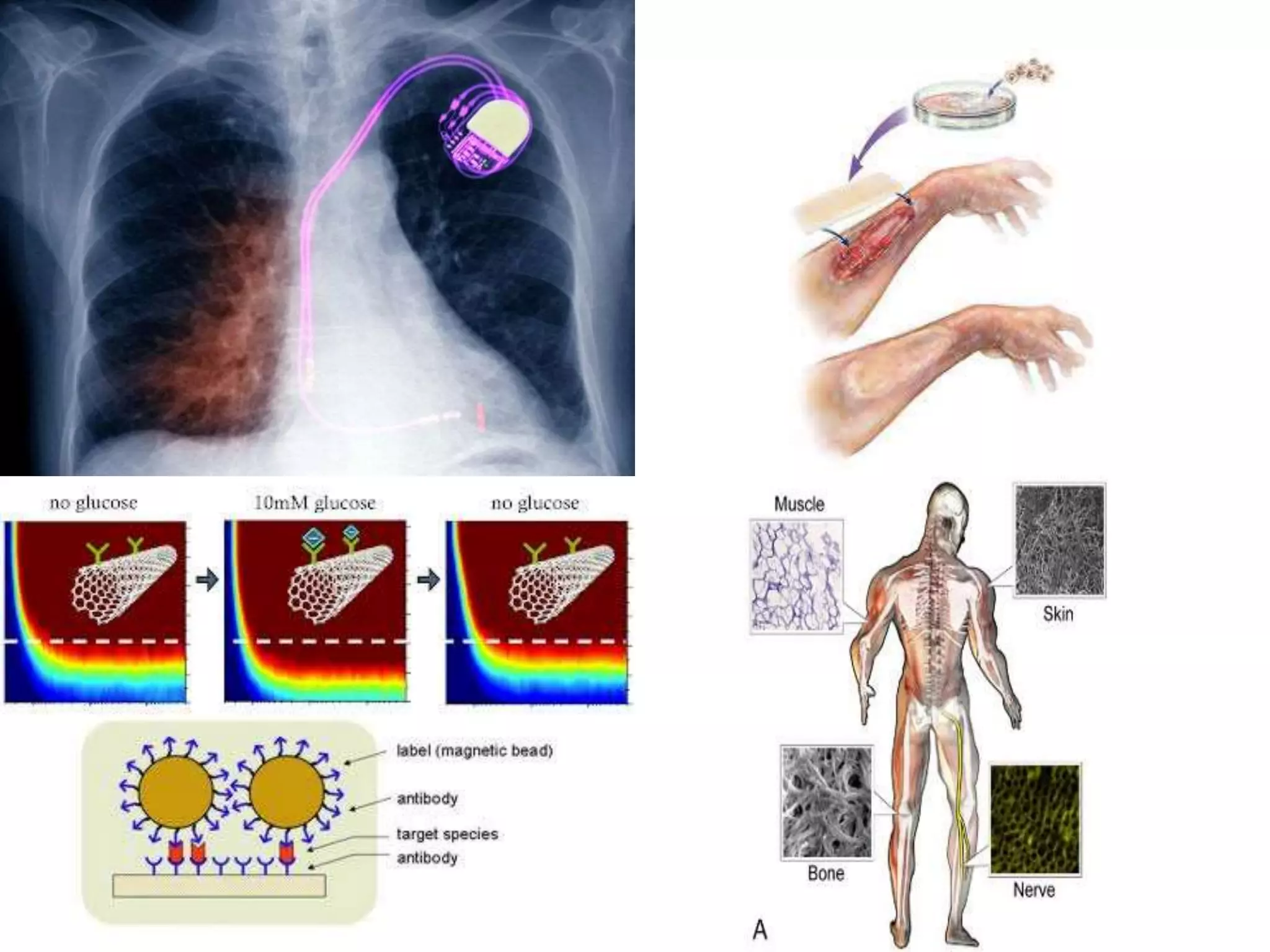
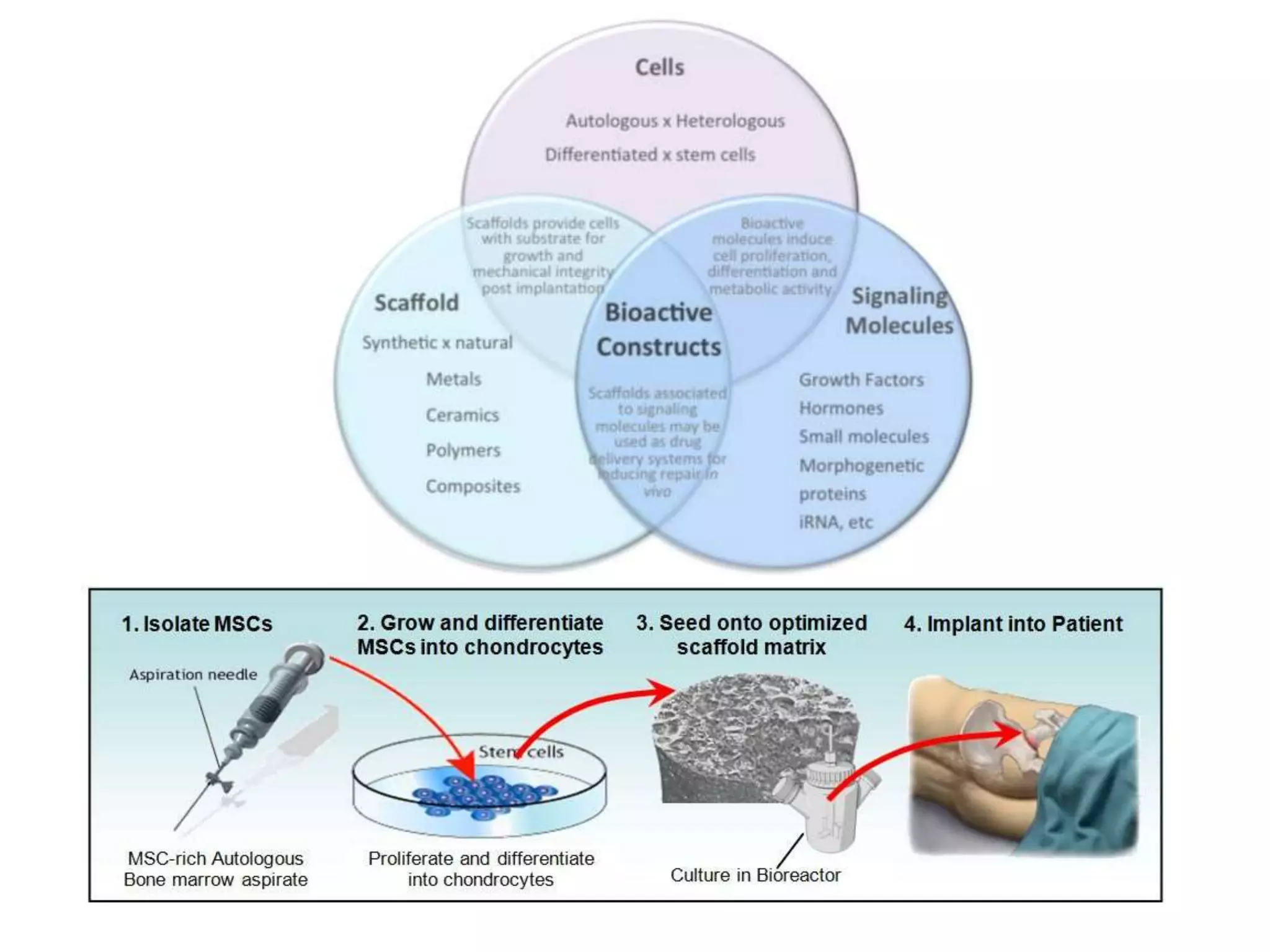
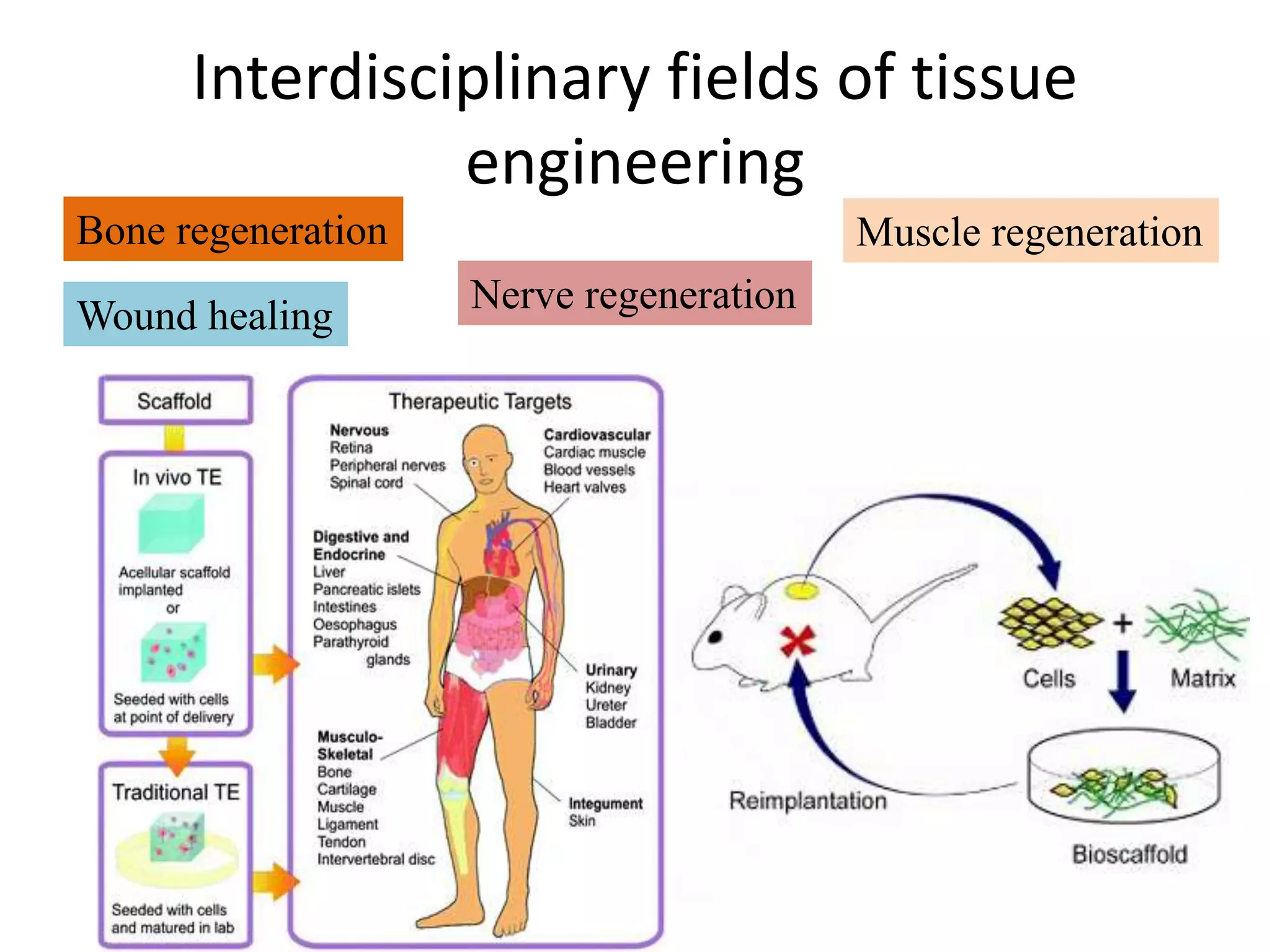
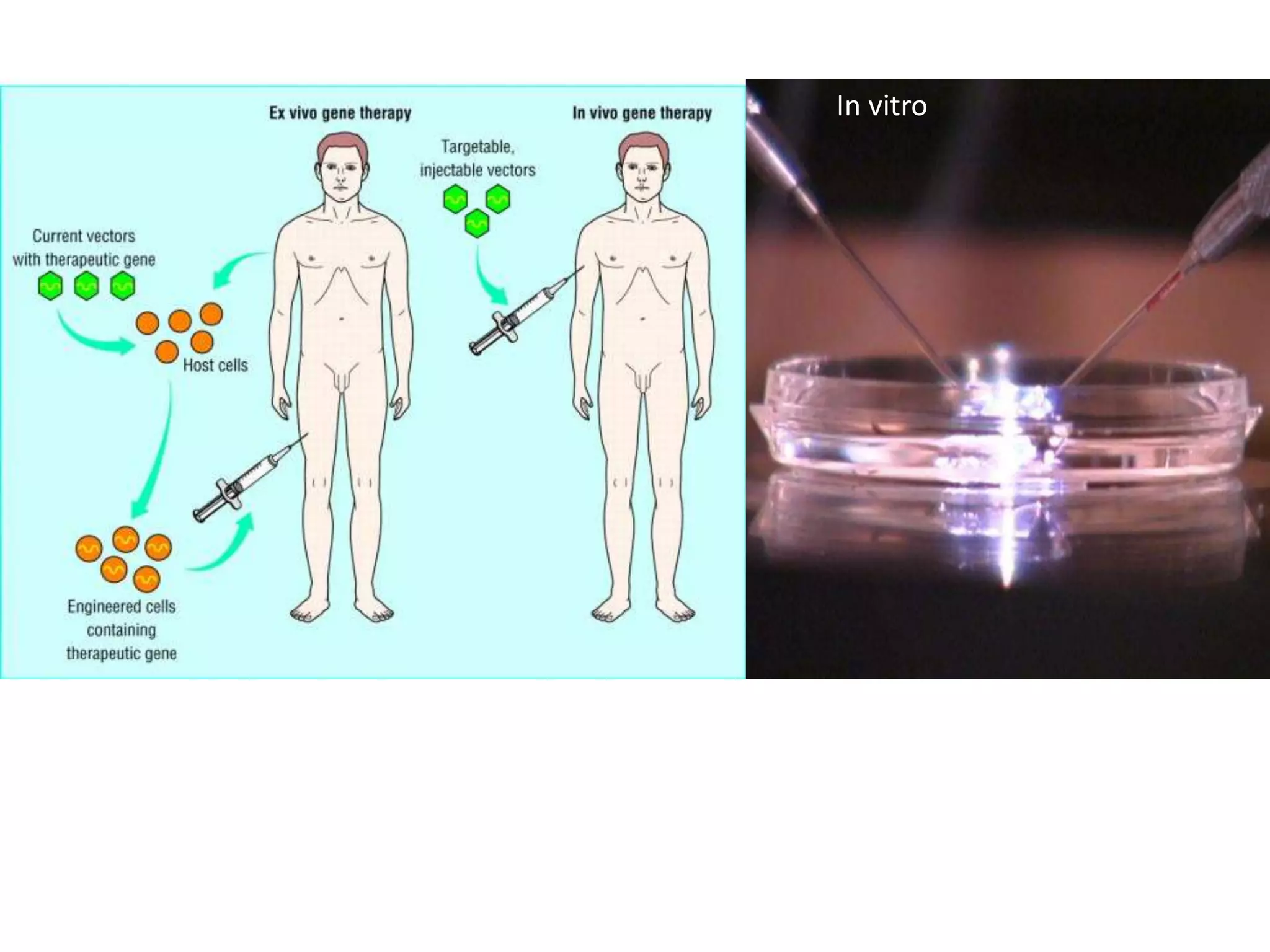
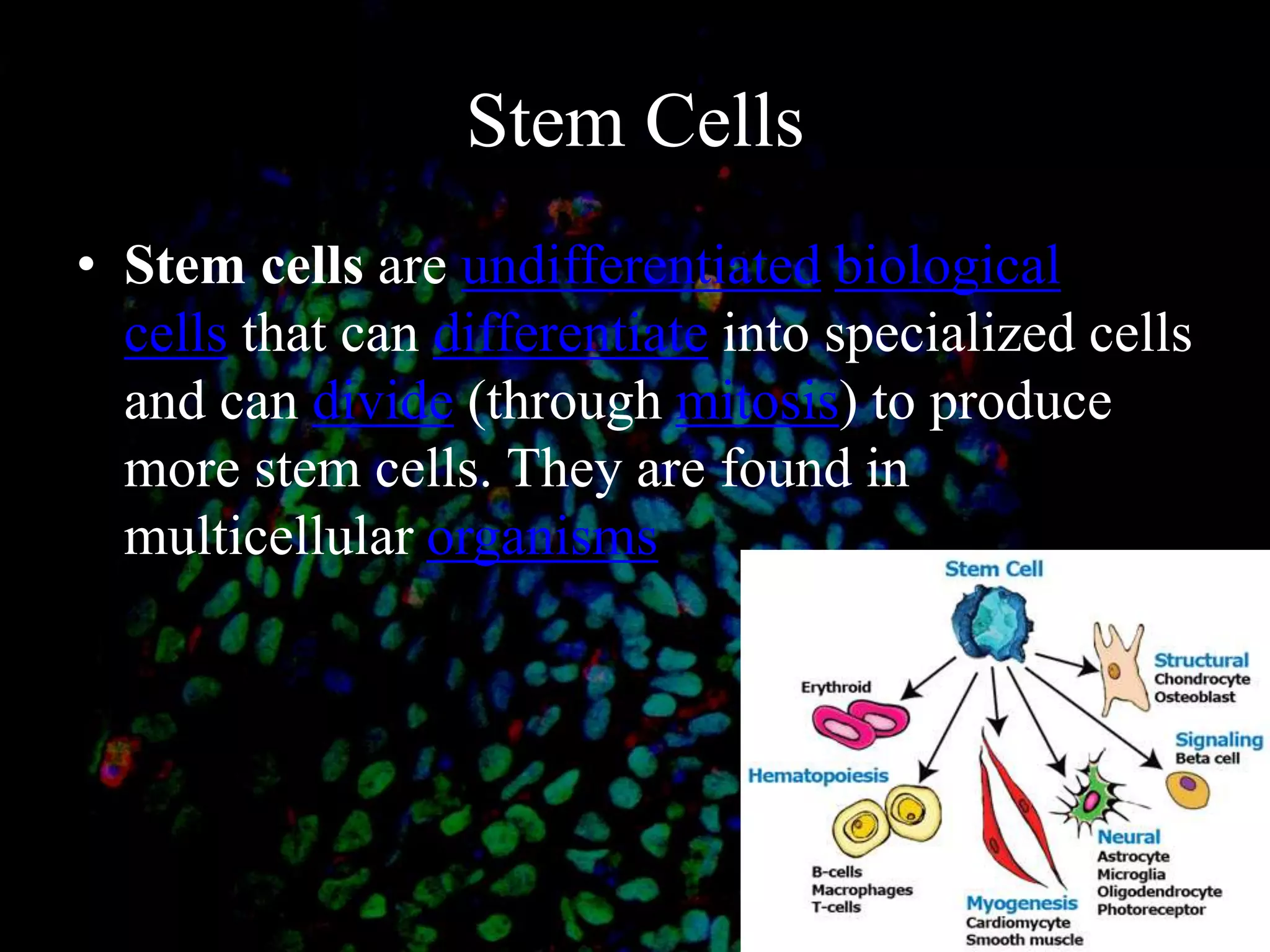
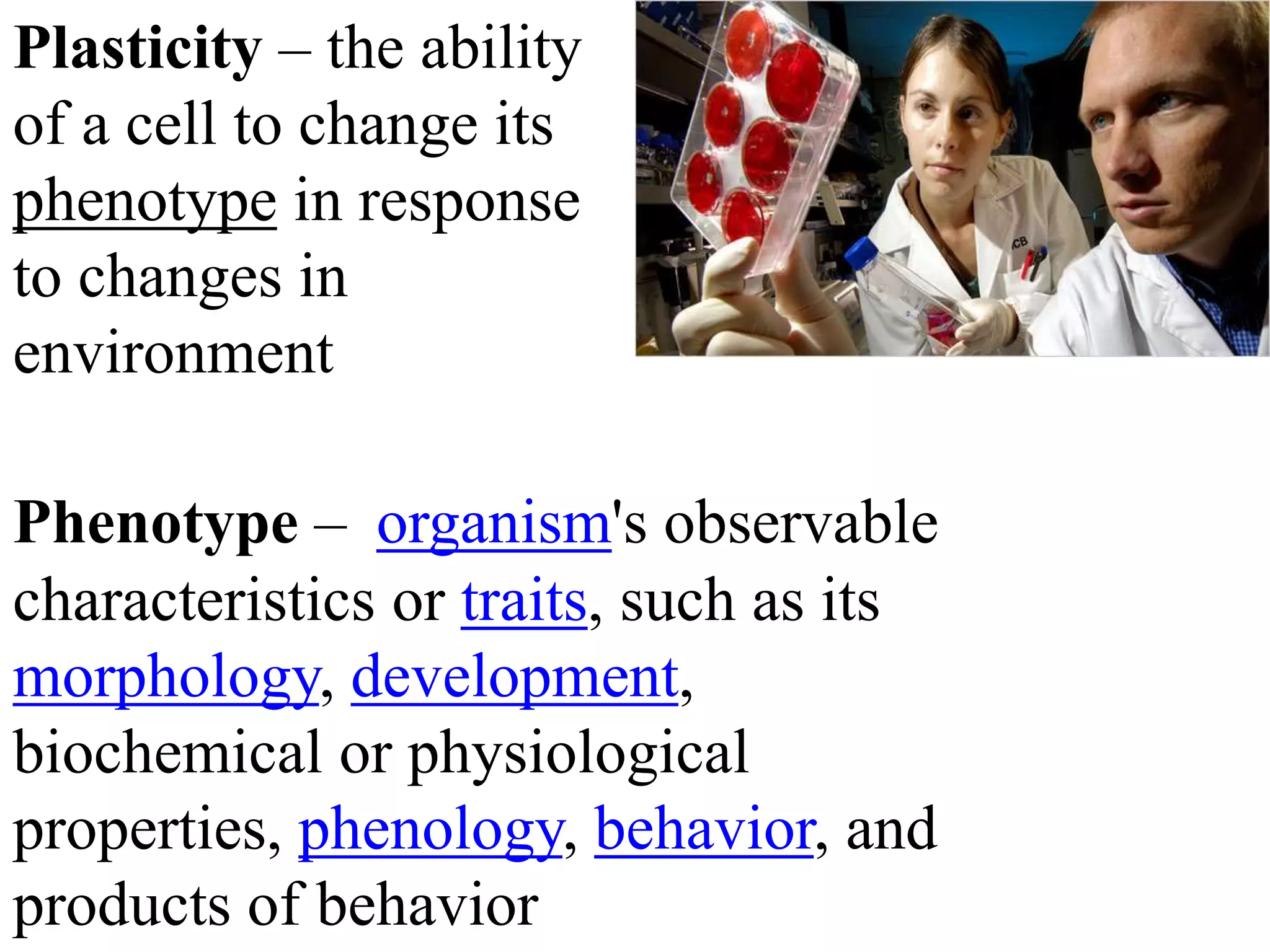
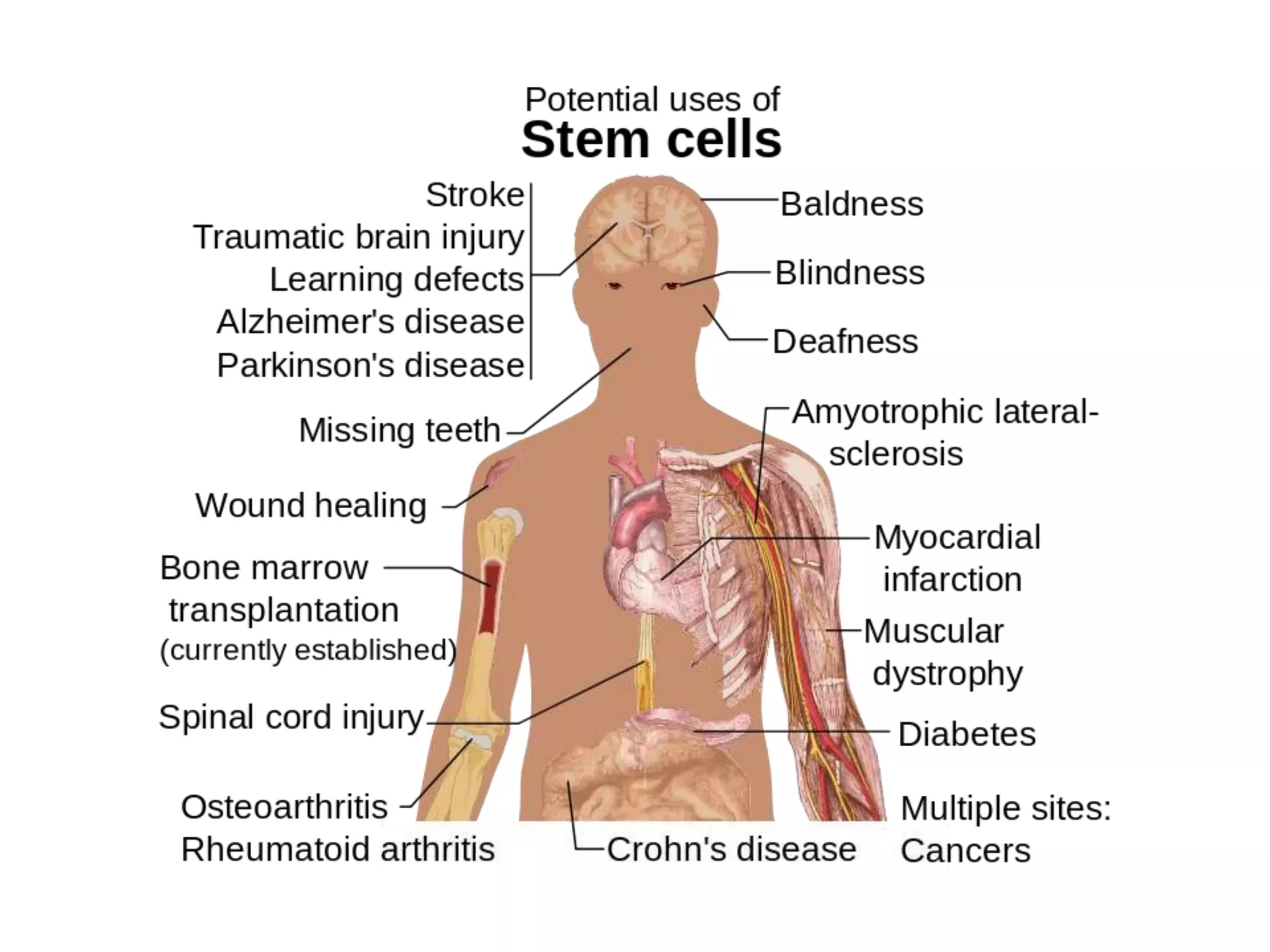
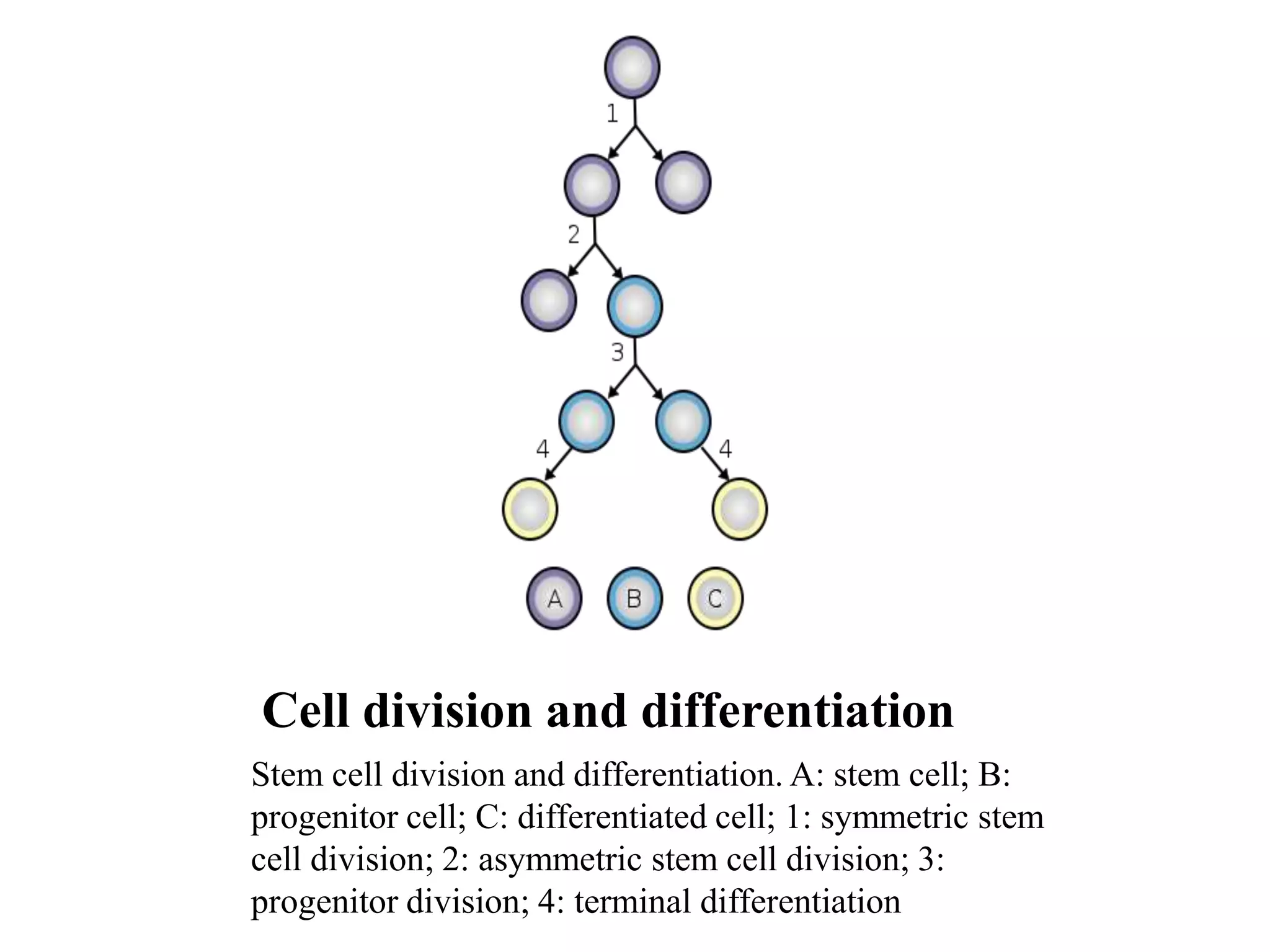
Tissue engineering is an interdisciplinary field that applies engineering and life science principles to develop biological substitutes that restore and maintain normal function. The goal is to repair, replace, and regenerate diseased tissue. Stem cells are undifferentiated cells that can differentiate into specialized cell types and divide to produce more stem cells. They have potential uses in tissue engineering but also risks like tumor formation that require further research.







![Cell potencies
• Potency specifies the differentiation potential (the potential to differentiate into
different cell types) of the stem cell.[4]
• Totipotent (a.k.a. omnipotent) stem cells can differentiate into embryonic and
extraembryonic cell types. Such cells can construct a complete, viable
organism.[4] These cells are produced from the fusion of an egg and sperm cell.
Cells produced by the first few divisions of the fertilized egg are also totipotent.[5]
• Pluripotent stem cells are the descendants of totipotent cells and can differentiate
into nearly all cells,[4] i.e. cells derived from any of the three germ layers.[6]
• Multipotent stem cells can differentiate into a number of cell types, but only those
of a closely related family of cells.[4]
• Oligopotent stem cells can differentiate into only a few cell types, such as lymphoid
or myeloid stem cells.[4]
• Unipotent cells can produce only one cell type, their own,[4] but have the property
of self-renewal, which distinguishes them from non-stem cells (e.g. progenitor
cells, muscle stem cells).](https://image.slidesharecdn.com/tissueengineeringbte4234class1-160812224926/75/Tissue-engineering-101-28-2048.jpg)