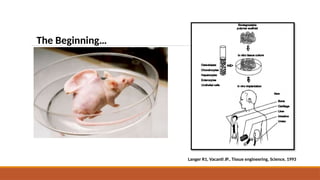
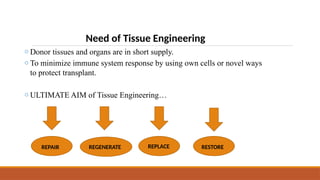
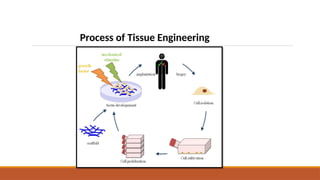
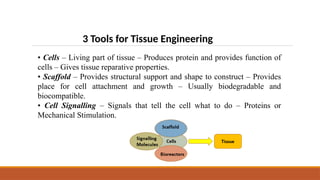
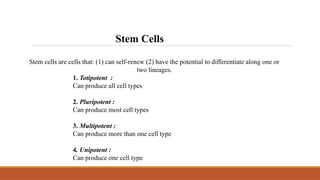
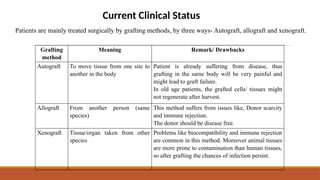
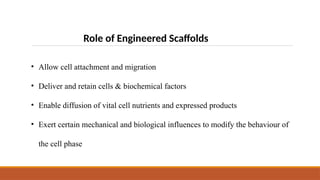
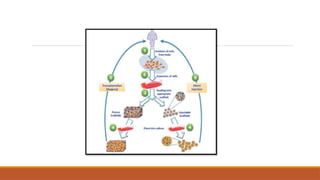
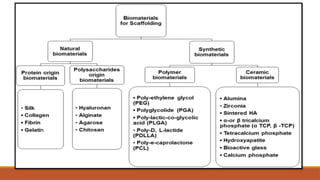
The seminar on tissue engineering presented by Sweta Naik discusses the interdisciplinary field focused on developing biological substitutes to restore or improve tissue functions through regenerative medicine. It highlights the importance of tools such as scaffolds, stem cells, and engineering techniques for effective tissue repair, while also addressing current clinical methods and their limitations. Key applications and properties of engineered scaffolds are outlined, emphasizing their role in medical advancements.