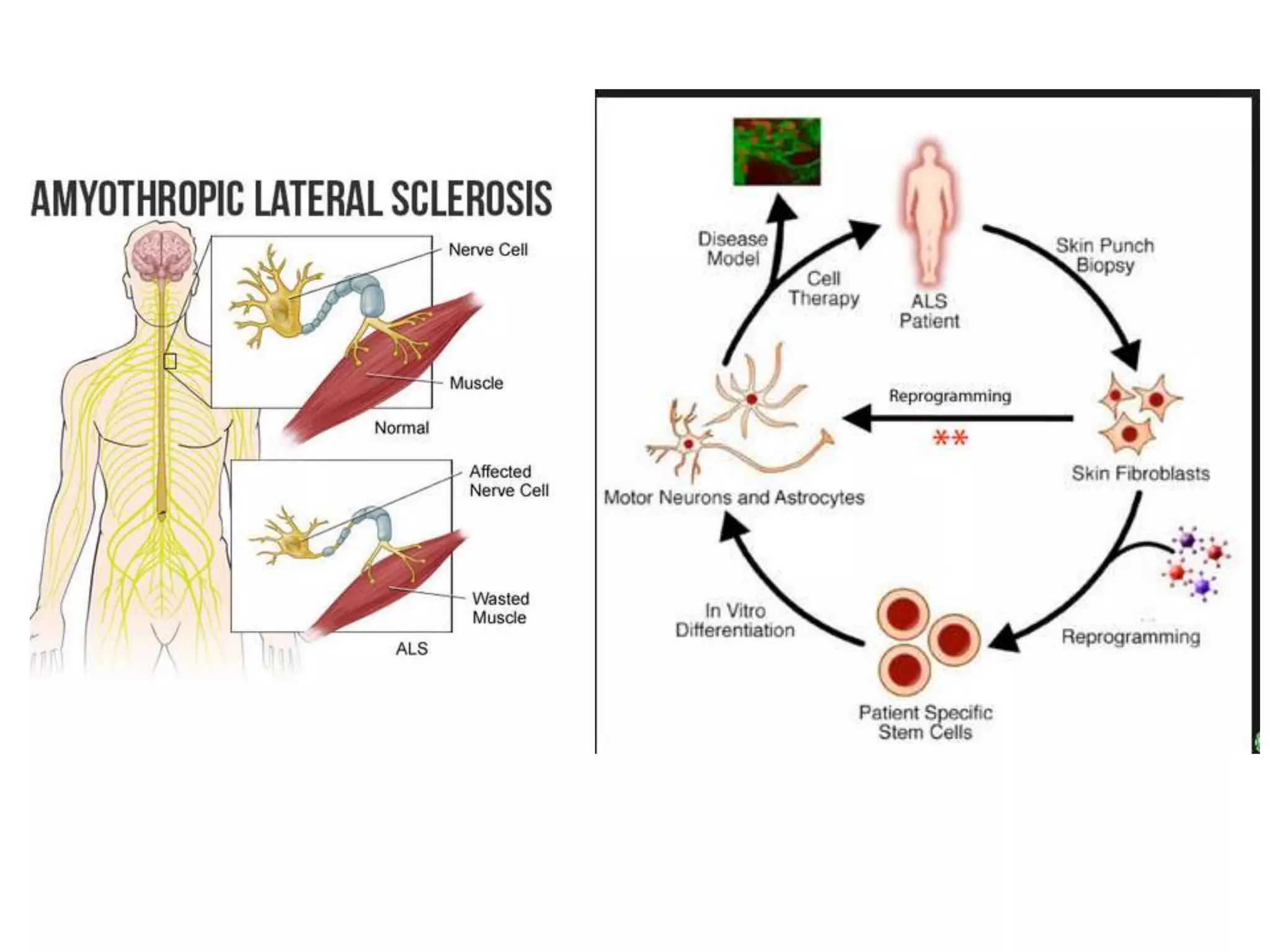
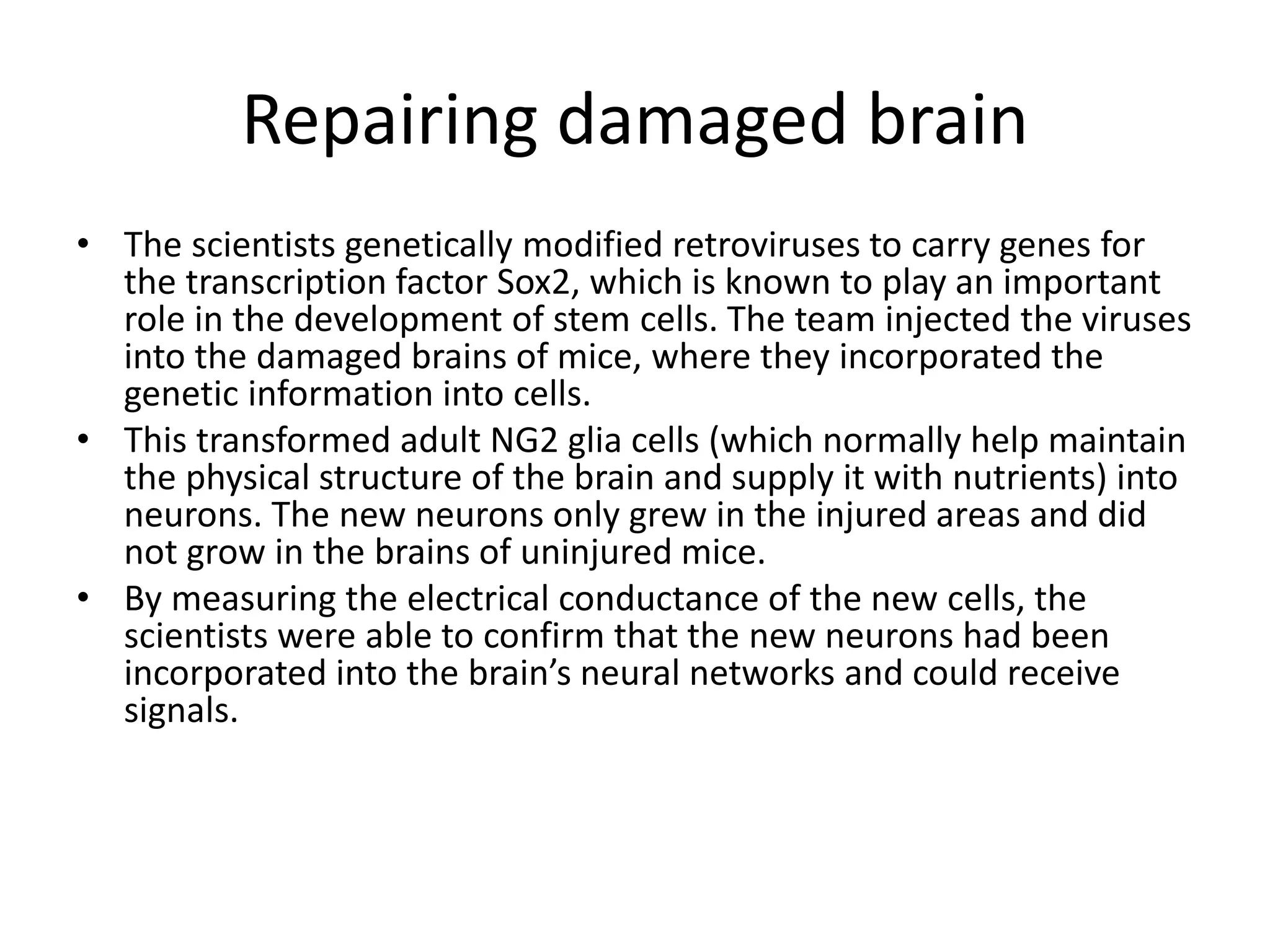
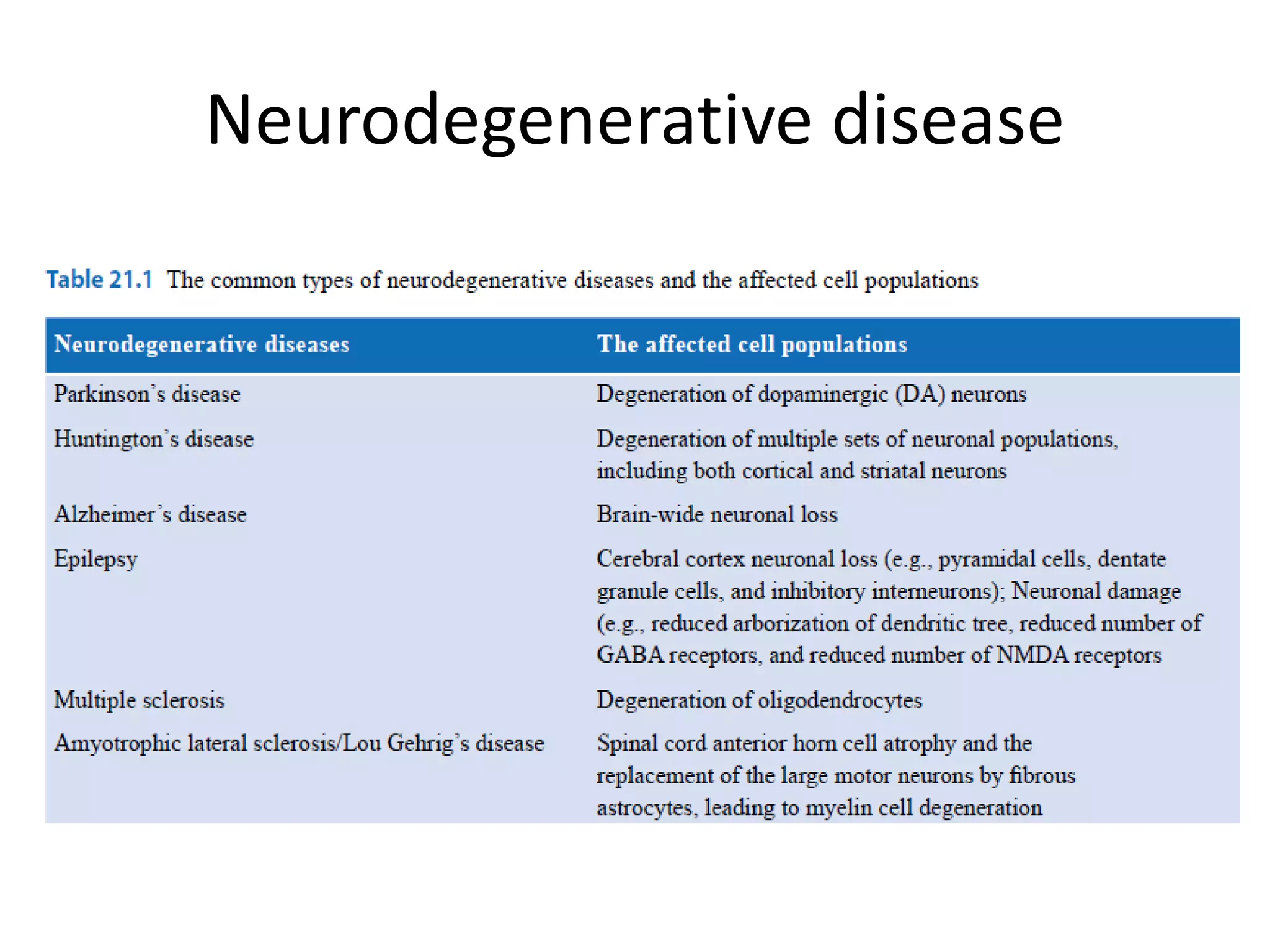
This document discusses nervous system injury and regeneration strategies. It describes how the nervous system is divided into the central and peripheral systems. Injury can occur via different factors and disrupts communication between neurons. In the central nervous system, axons do not regenerate naturally due to inhibitors at injury sites. Tissue engineering strategies aim to use guidance channels, cell recovery, drug delivery, and electrical stimulation to promote regeneration, especially by transforming glial cells into neurons using genetic modification in mice models. Advances in developmental biology and tissue engineering principles can mimic cues to direct neural tissue regeneration.





![Tissue Engineering Strategies
for Nervous System Repair
• Use of nerve guidance channels is a promising
nerve graft alternative.
• A variety of channels presenting physical,
chemical, mechanical, and biological guidance
cues to regenerating nerves have been
developed with the potential for nerve repair
following PNS and spinal cord injuries, and
neurodegenerative pathologies [e.g.,
Parkinson’s disease (PD)]](https://image.slidesharecdn.com/nervoussysteminjuryandregeneration-170313043254/75/Nervous-system-injury-and-regeneration-10-2048.jpg)