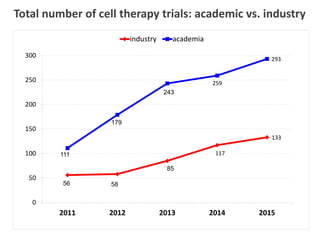
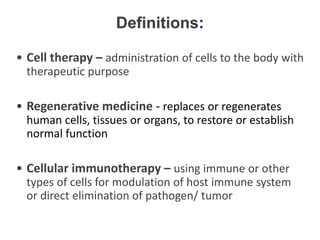
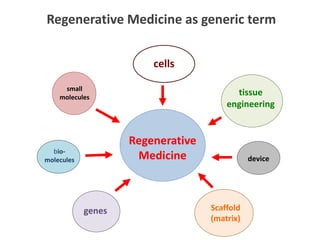
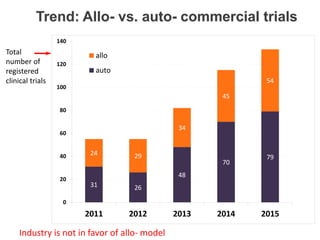
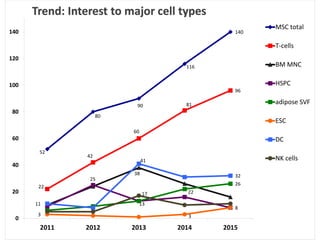
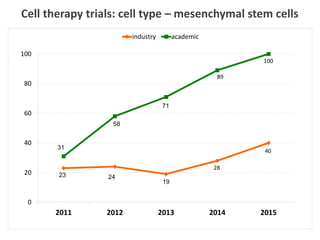
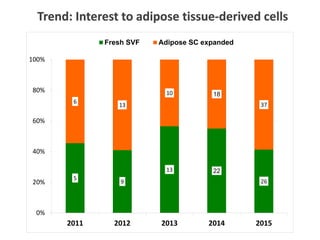
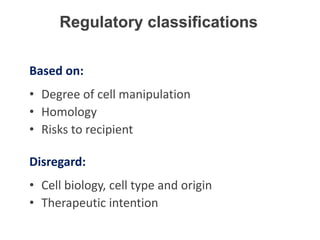
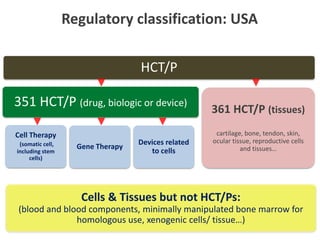
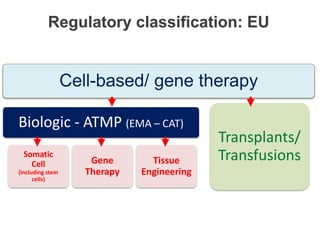
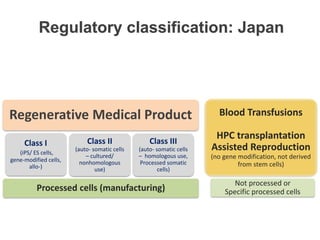
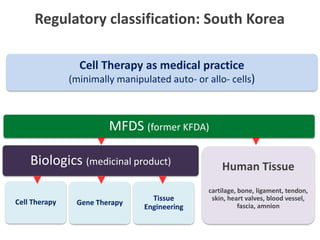
This document discusses definitions, classifications, and trends related to cell therapy. It defines cell therapy as the administration of cells to the body for therapeutic purposes and regenerative medicine as replacing or regenerating tissues to restore function. Classifications of cell therapies include immunocellular vs regenerative approaches and autologous vs allogeneic cell sources. The trends show growing interest in immunocellular therapies and use of mesenchymal stem cells and adipose tissue-derived cells. Regulatory classifications of cell therapies vary between countries and agencies and certain cell therapies can be difficult to clearly define, creating potential confusion.