This document provides an overview of key concepts related to water quality parameters and perspectives. It discusses the following key points in 3 sentences:
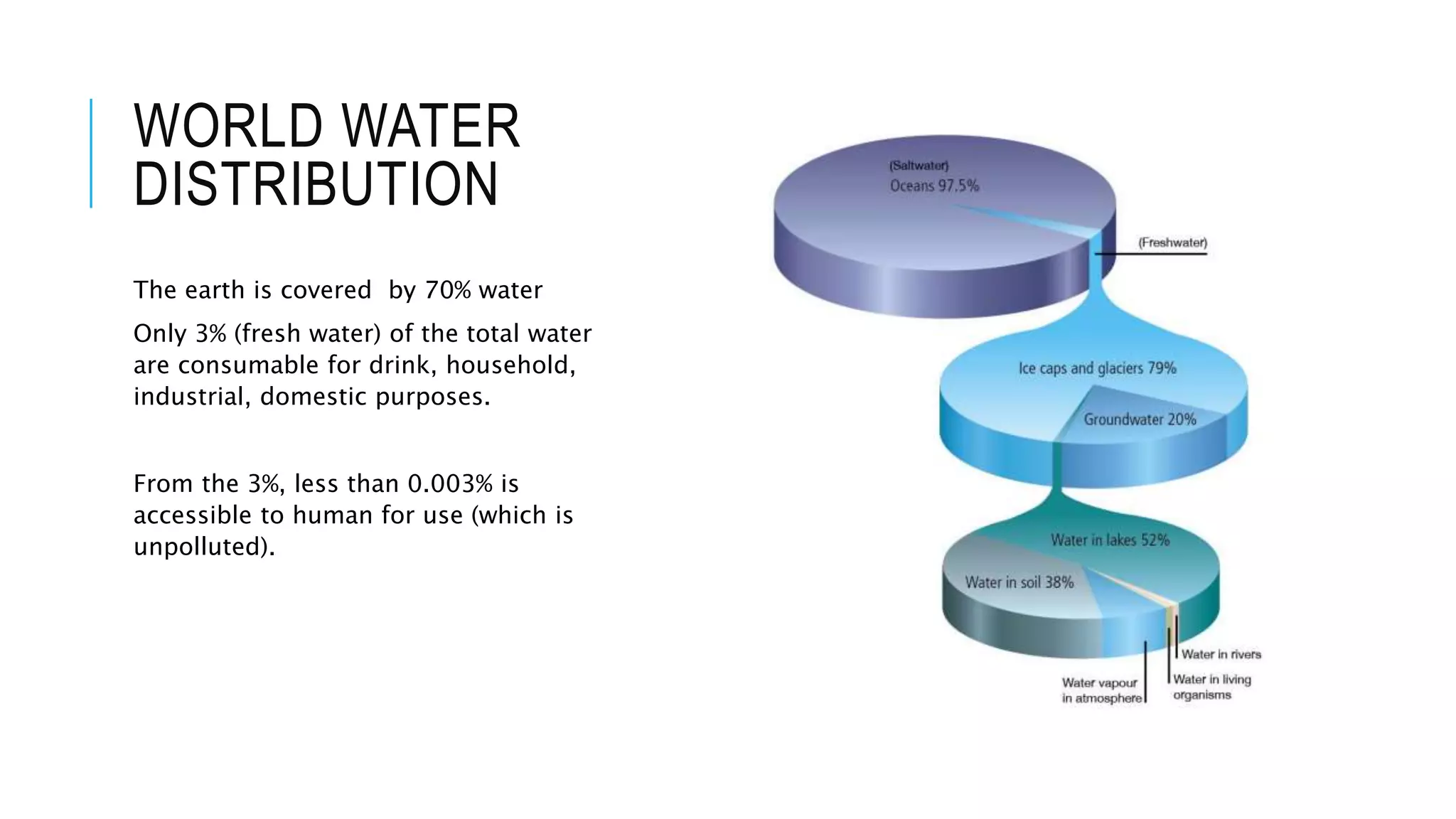
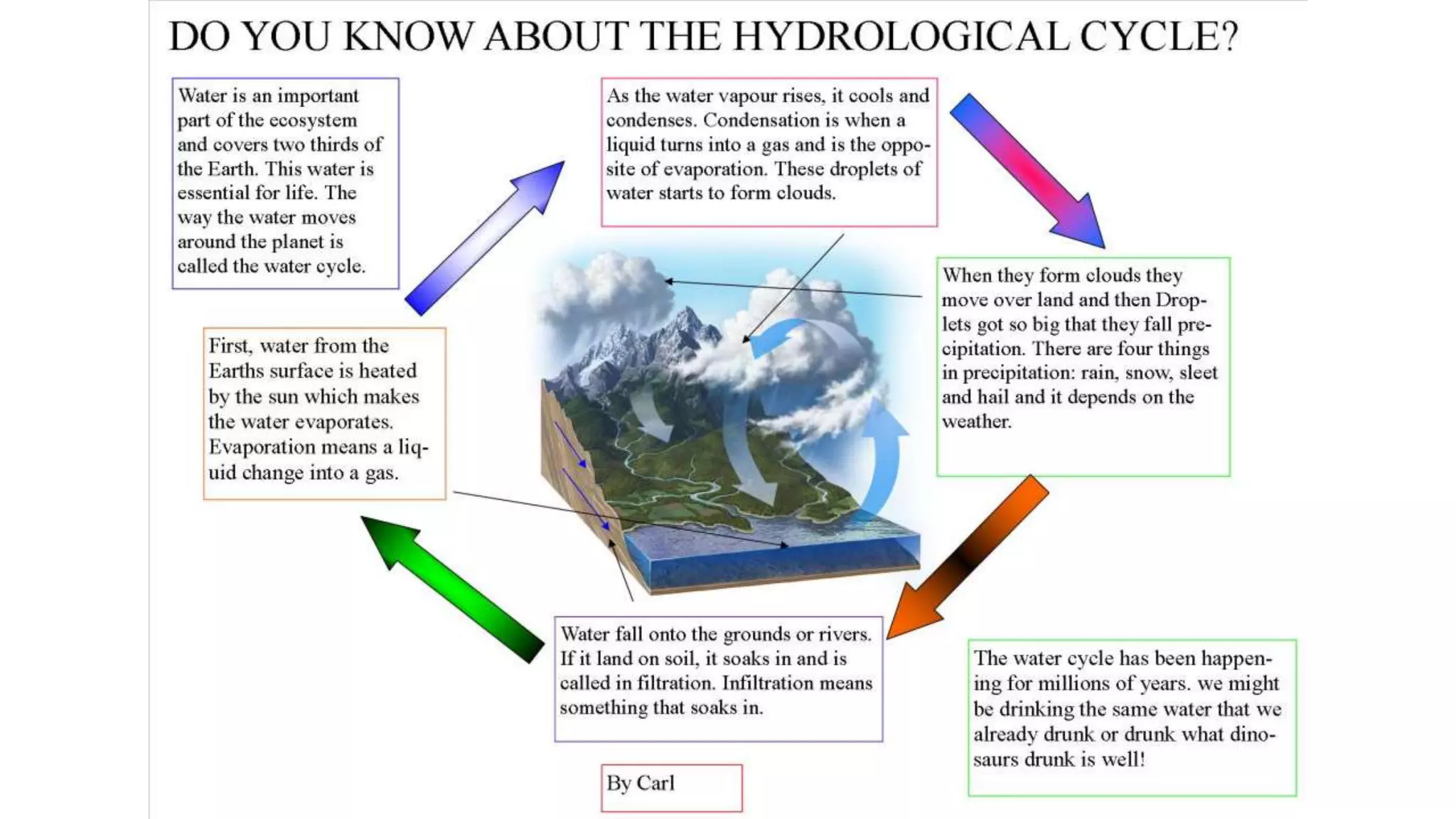
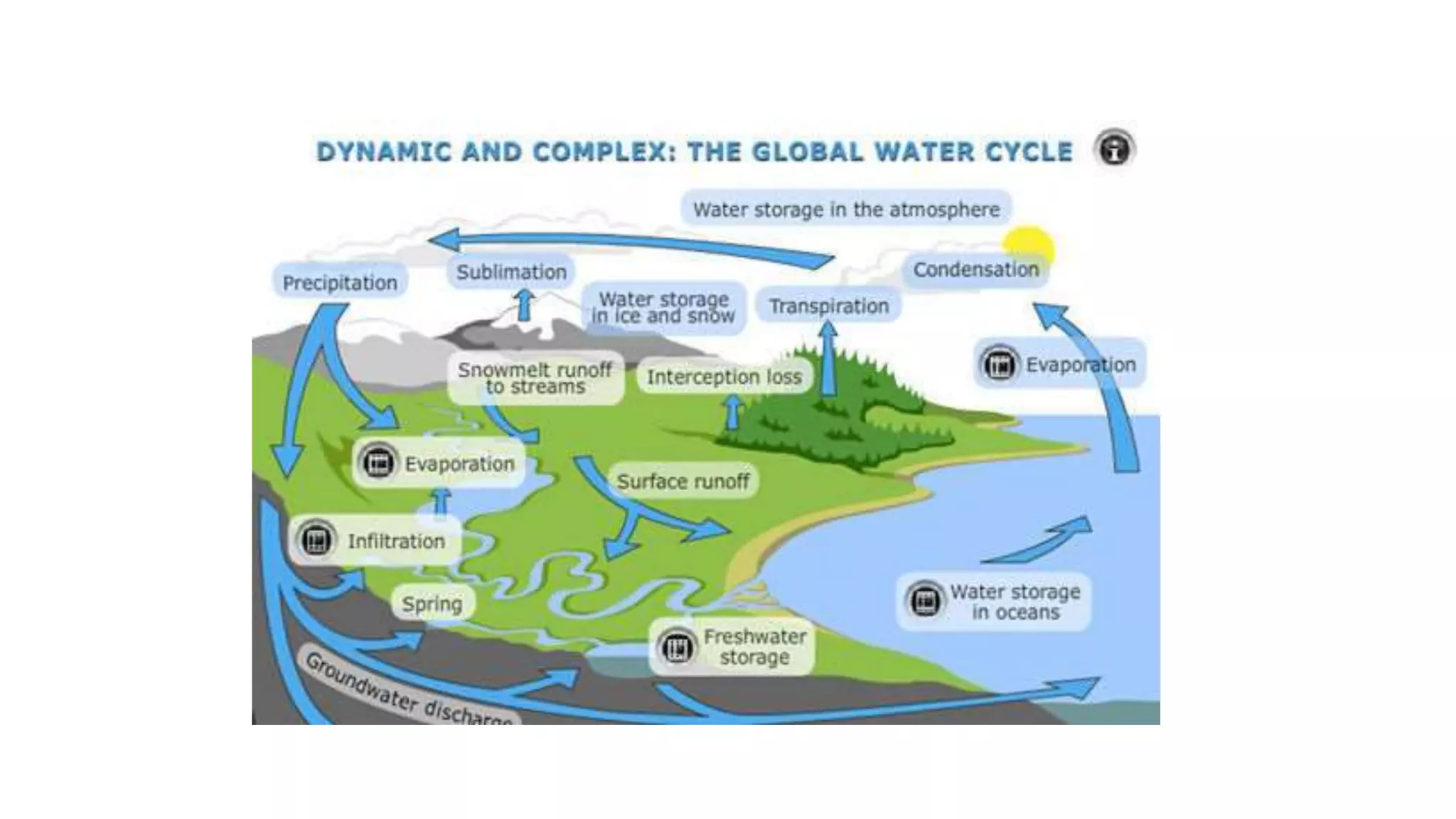
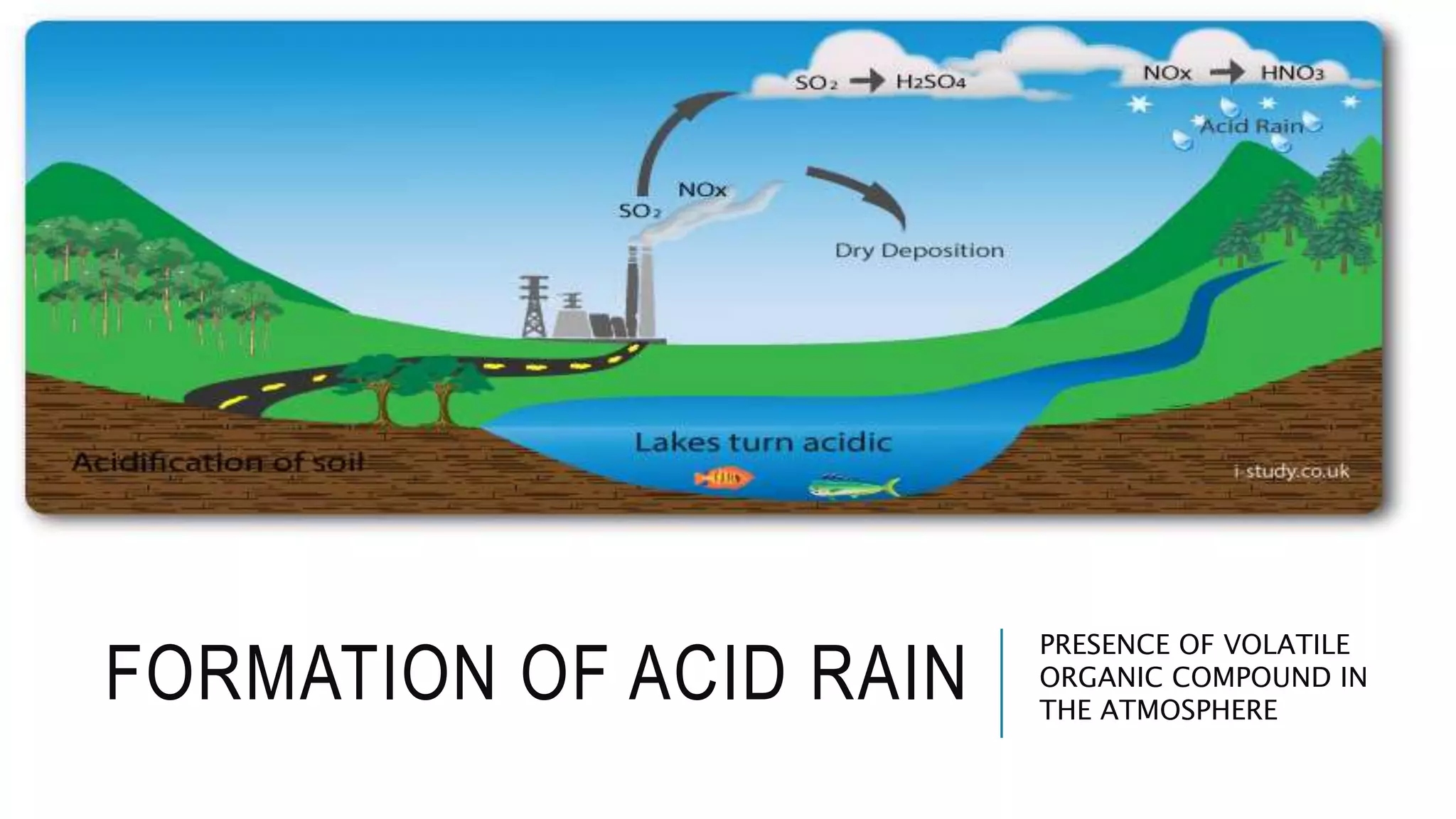
- Only 3% of the total water on Earth is fresh water that is consumable for human use, and less than 0.003% of total water is accessible to humans in an unpolluted state. Water sustainability is essential to human civilization and development. Various human activities contribute to water contamination through the hydrologic cycle.
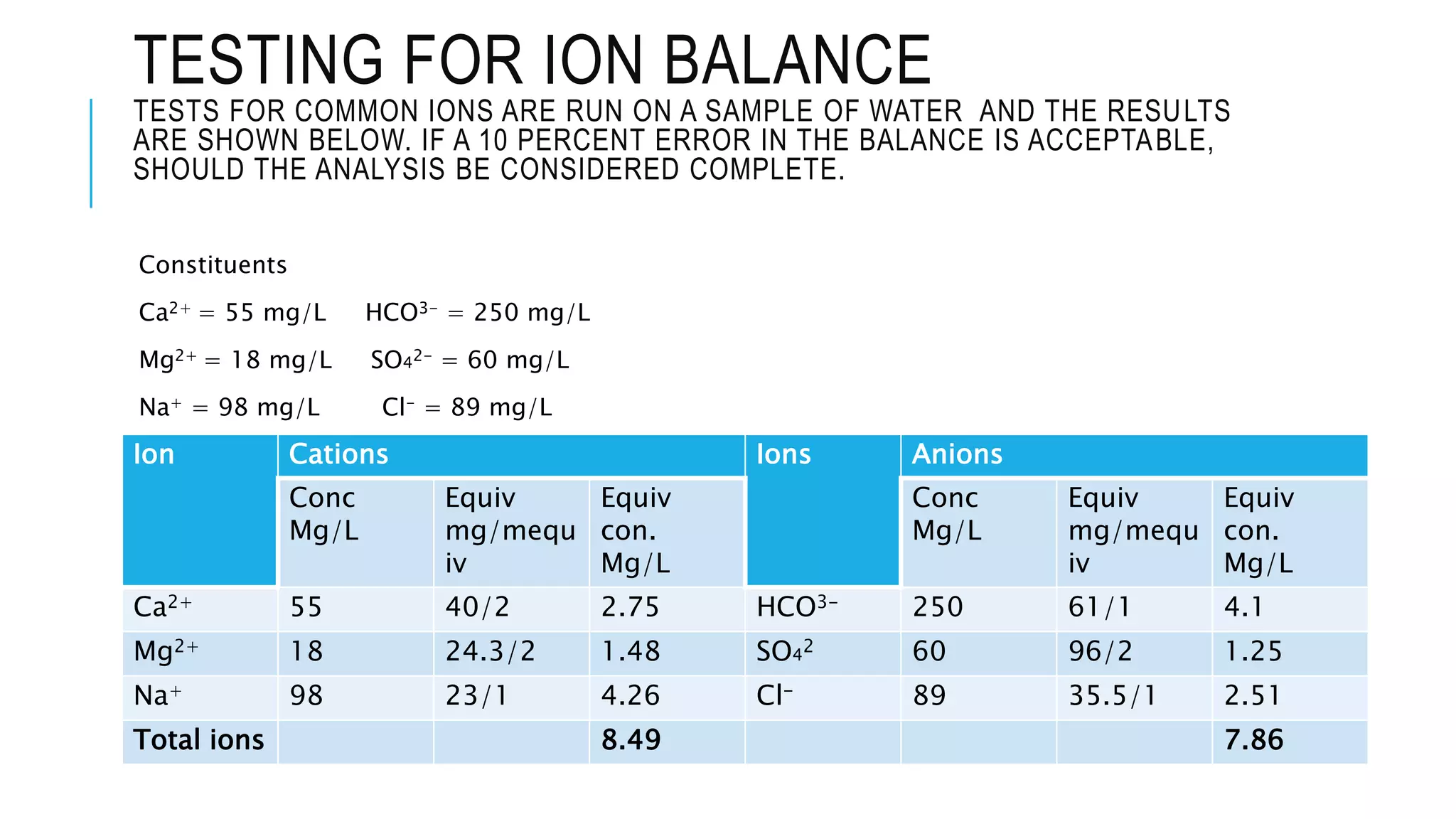
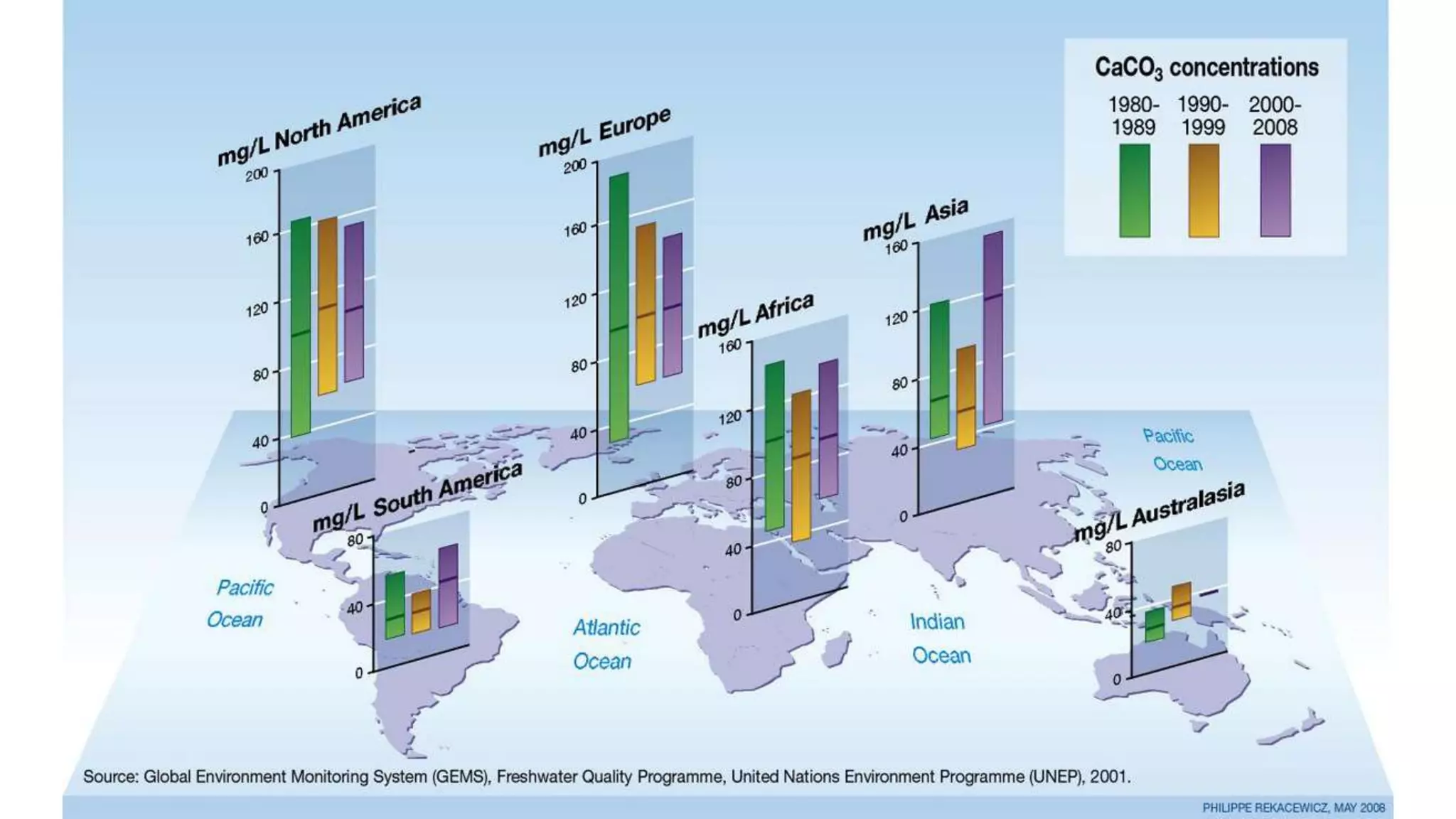
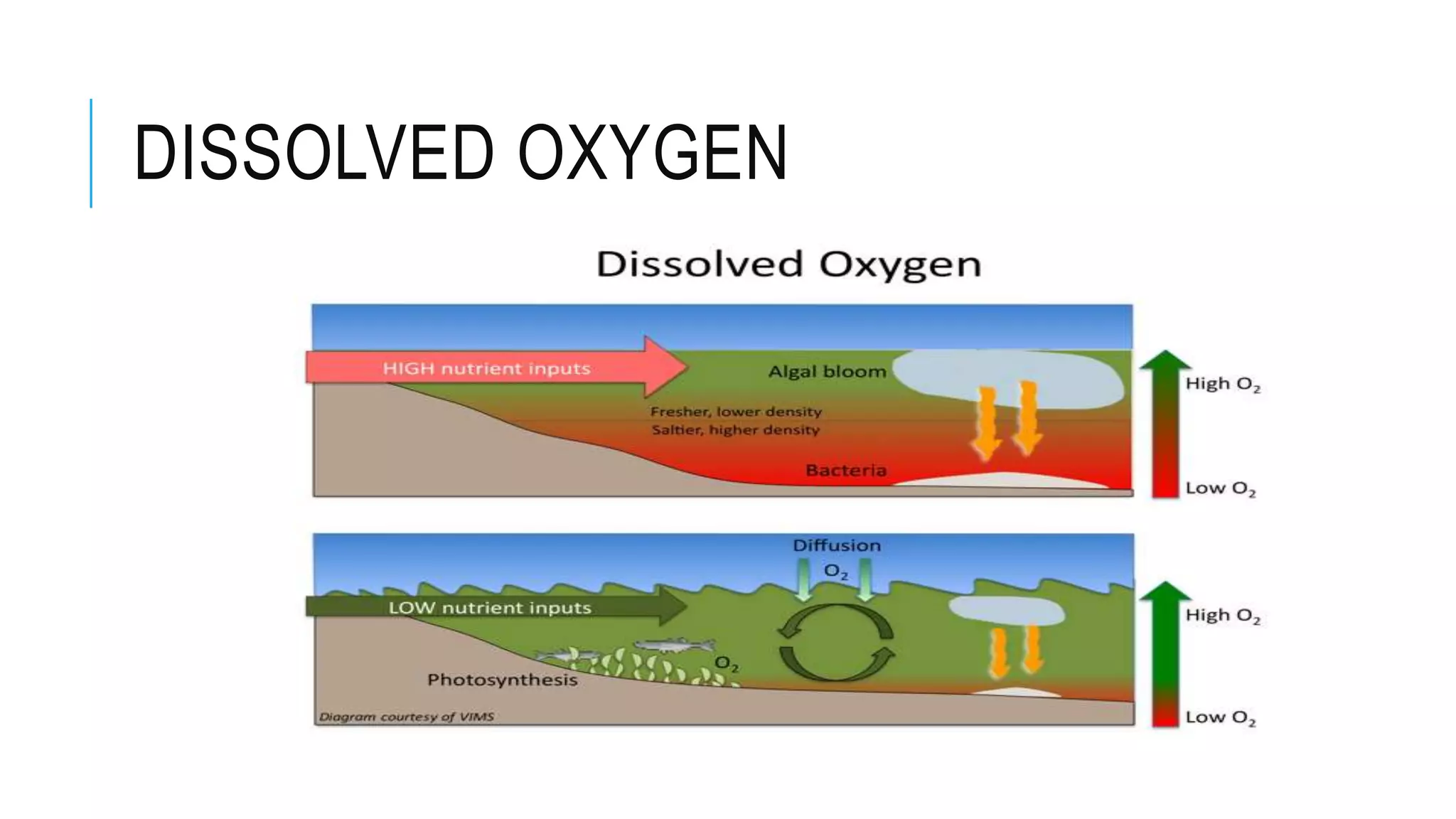
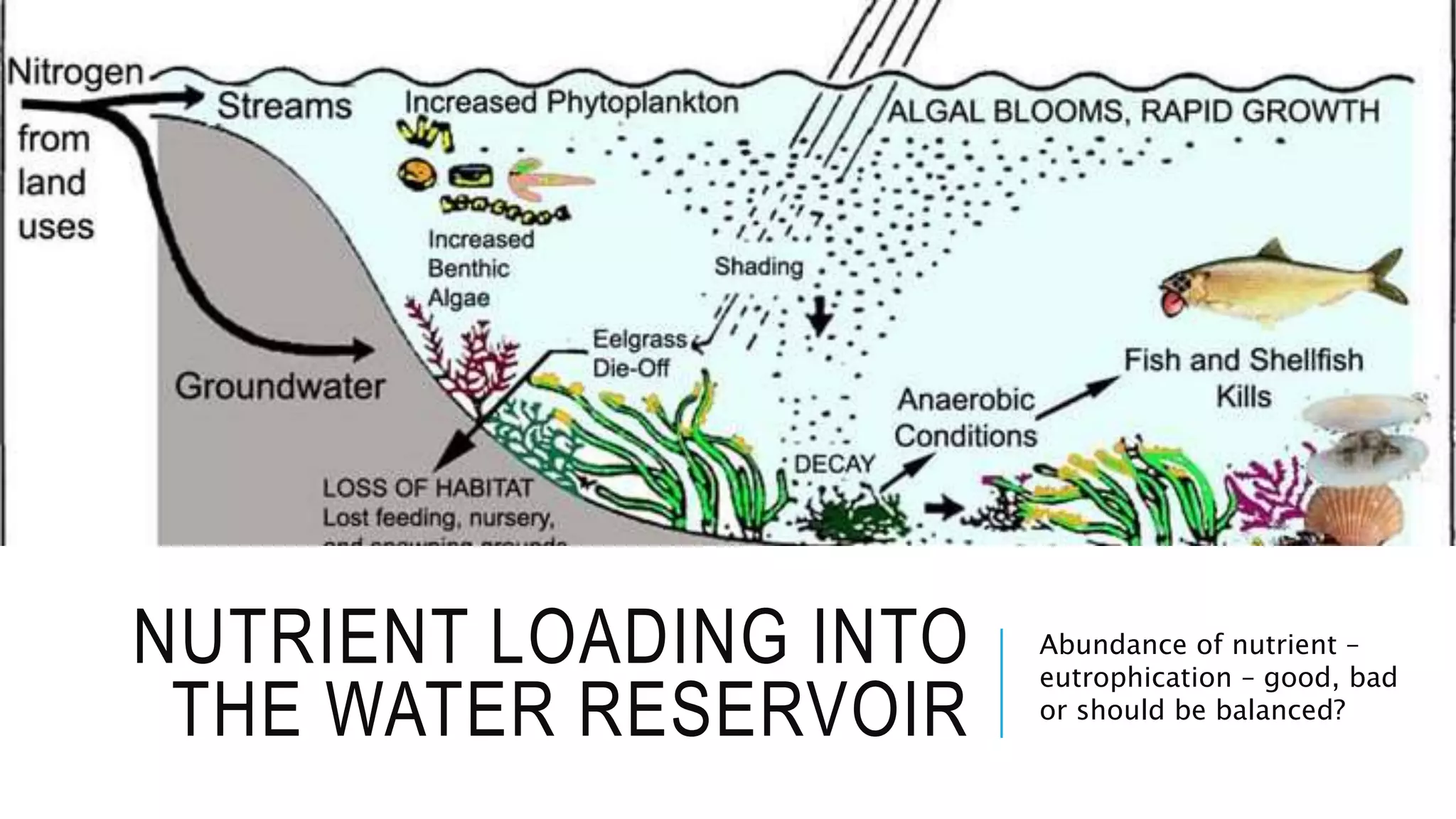
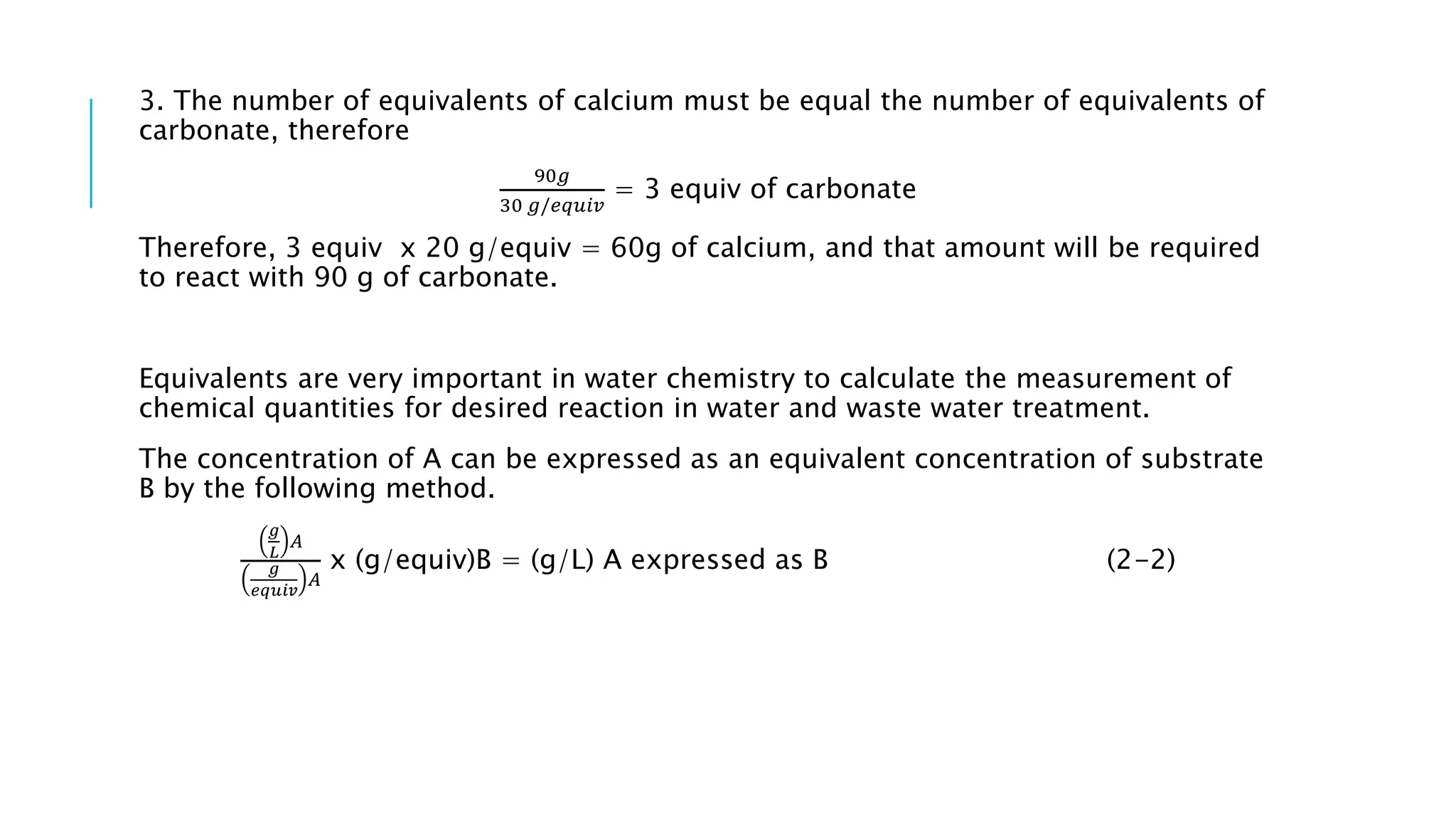
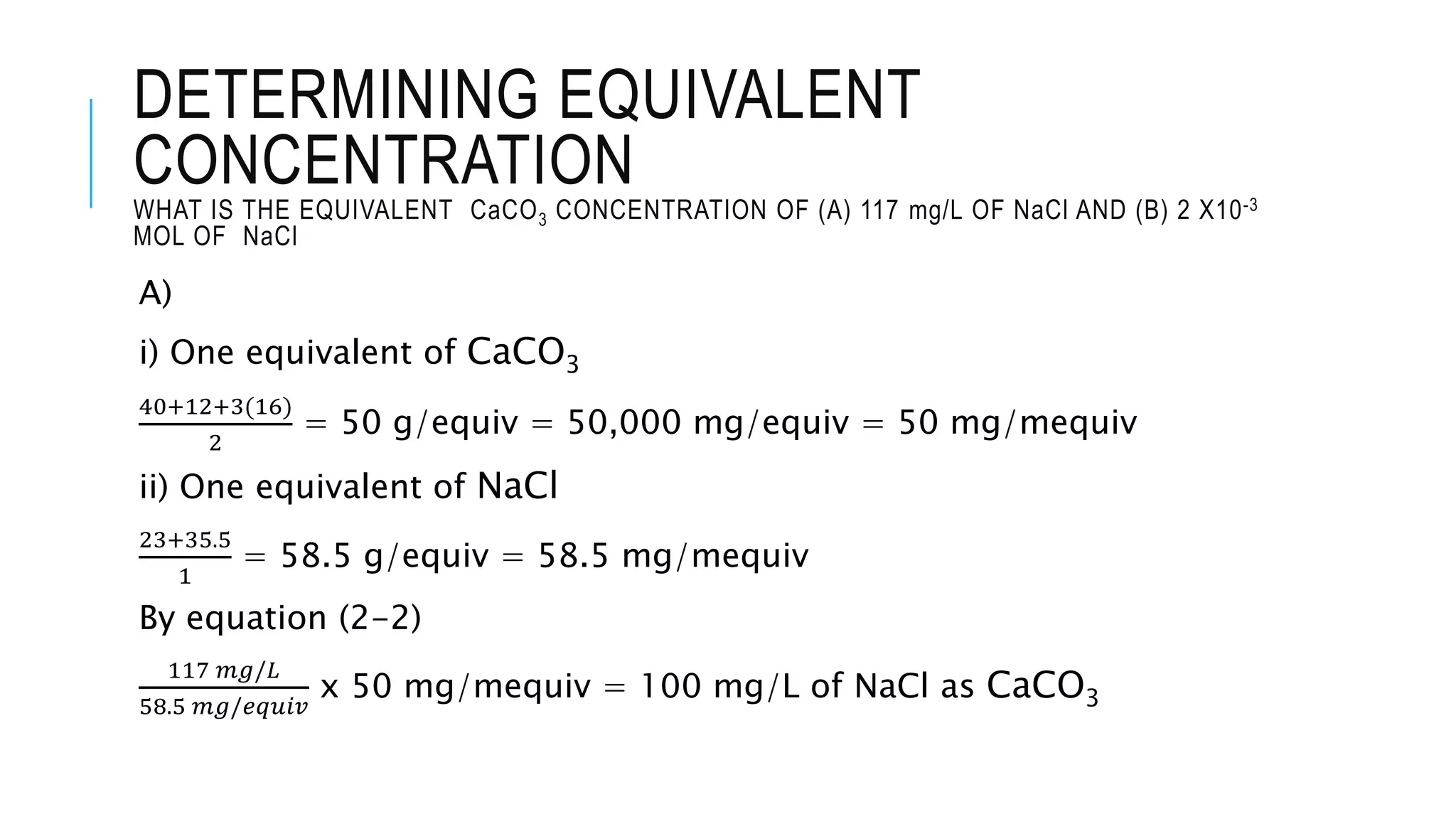
- Chemical parameters related to water quality include total suspended solids, alkalinity, hardness, metals, nutrients, and other factors. The chemical properties of water are determined by the dissolution of substances and the formation of ions, and concepts like moles,

















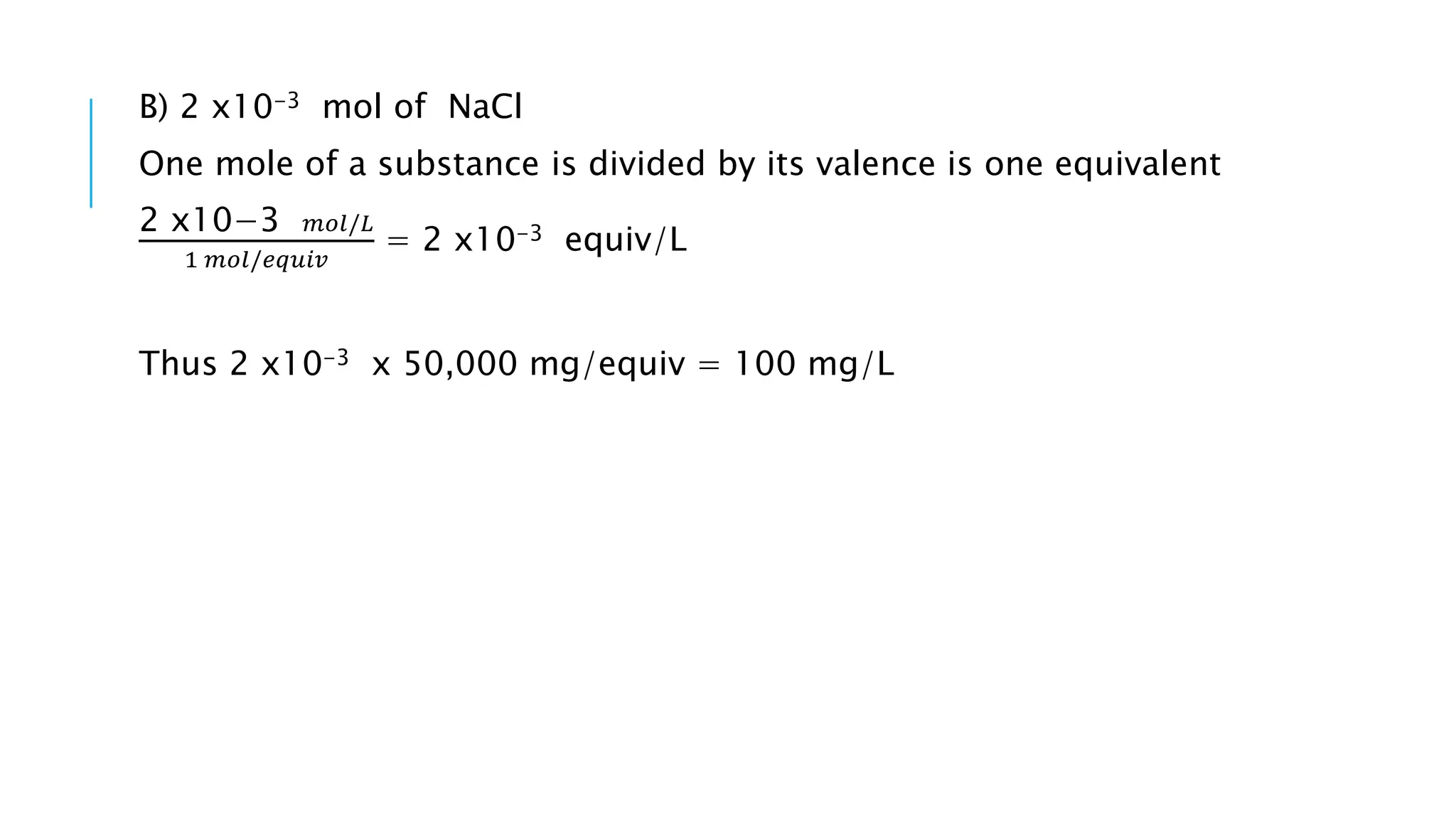
![The solid form NaCl may be dissociating into its ionic components (dissolution) or
recombine to form solid (precipitation)
Conditions of equilibrium can be expressed by the mass action equation. For
generalized reaction
AxBy ↔ xA + yB
Solid compound Ionic compound
The mass action equation is
[𝐴] 𝑥[𝐵] 𝑦
[𝐴 𝑥 𝐵 𝑦]
= K
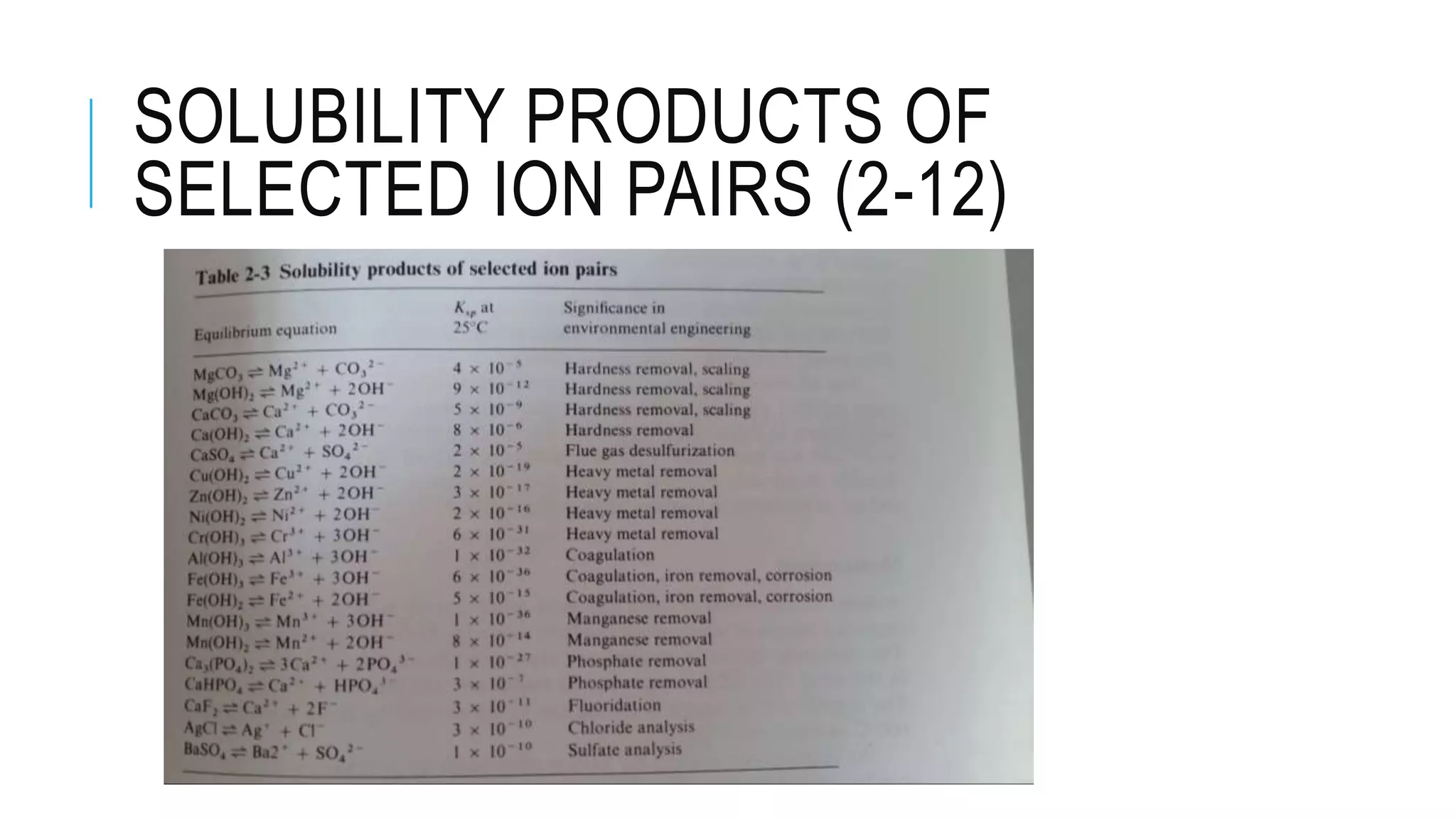
K value is an equilibrium constant for a given substance in pure water at
a given temperature.
[𝐴 𝑥 𝐵𝑦] = 𝐾𝑠 = constant and [𝐴] 𝑥
[𝐵] 𝑦
= K𝐾𝑠 = 𝐾𝑠𝑝
𝐾𝑠𝑝 is known as solubility product for the ion pair](https://image.slidesharecdn.com/chapter2-160822191407/75/Water-Quality-and-System-in-Environmental-Engineering-31-2048.jpg)
![DETERMINING EQUILIBRIUM
CONCENTRATIONS
The solubility product for the dissociation of Mg(OH)2 is shown in Table 2-3 as
9x10-12 Determine the concentration of Mg2+ and OH -at equilibrium, expressed
as miligrams per liter of CaCO3
Solution
1. Write the equation for the reaction
Mg(OH)2 ↔ Mg2+ and 2OH-
2. The solubility of the product equation becomes
[Mg2+ ] [OH- ]2 = 9x10-12
If x is the number of moles of Mg2+ resulting from the dissociation, then OH- is
equal to 2x, Therefore](https://image.slidesharecdn.com/chapter2-160822191407/75/Water-Quality-and-System-in-Environmental-Engineering-32-2048.jpg)
![[x] [2x]2 = 9x10-12
4x3 = 9x10-12
X = 1.3𝑥10 − 4 𝑚𝑜𝑙/𝐿 = Mg
2X = 2.6x10-4 mol/L = OH
3.
1.3x10−4 mol/L
0.5 𝑚𝑜𝑙/𝑒𝑞𝑢𝑖𝑣
x 50,000 mg/equiv = 13.0 mg/L of Mg as CaCO3
4.
2.6x10−4 mol/L
1𝑚𝑜𝑙/𝑒𝑞𝑢𝑖𝑣
x 50,000 mg/equiv = 13.0 mg/L of Mg as CaCO3](https://image.slidesharecdn.com/chapter2-160822191407/75/Water-Quality-and-System-in-Environmental-Engineering-33-2048.jpg)