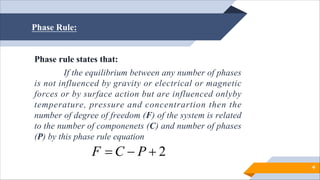
The document discusses the phase rule, which states that for a system in equilibrium that is influenced only by temperature, pressure, and concentration, the number of degrees of freedom (F) equals 2 plus or minus the number of phases (P) minus the number of components (C). It defines key terms like phase, component, and degree of freedom. It provides examples of applying the phase rule to systems with one, two, or three components. The phase rule helps predict a system's behavior under different conditions and indicates how systems with the same degrees of freedom will behave similarly.