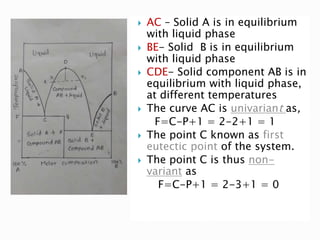
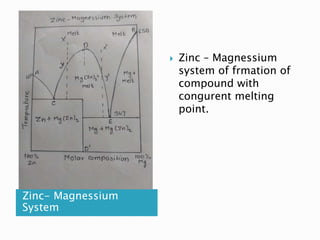
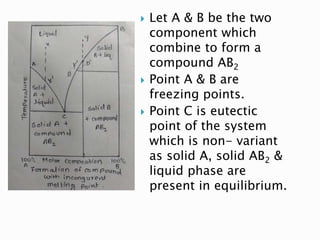
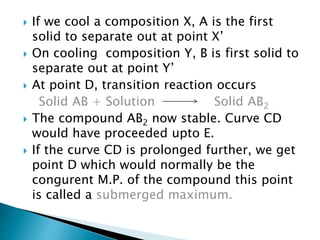
The document discusses different types of compound formations including compounds with congruent and incongruent melting points. It defines key terms like phase, component, degree of freedom, and explains concepts like the phase rule and how it relates the number of components, phases, and degrees of freedom in a system. Examples of systems forming compounds with congruent melting points like zinc-magnesium and systems forming compounds with incongruent melting points like picric acid-benzene are provided along with diagrams showing their phase diagrams.