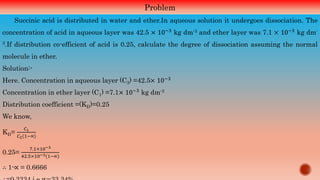
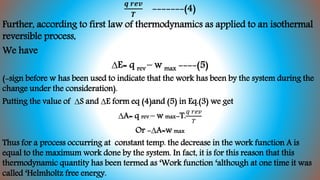
The document explains thermodynamic concepts such as Gibbs's and Helmholtz free energy, focusing on their definitions, relations to entropy, and their significance in determining the maximum work obtainable from thermodynamic processes. It includes mathematical formulations demonstrating how free energy changes relate to enthalpy, temperature, and chemistry, asserting that negative changes in free energy indicate spontaneous reactions. Additionally, it discusses applications of the Gibbs-Helmholtz equation for calculating thermodynamic properties in reactions and energy changes.






























![VAN’T HAFF REACTION ISOTHERM
Let us consider following reaction at temperature T(K) aA(g)+bG(g) lL(g)+mM(g)
free energy change (∆G) for this reaction is give by
∆G= 𝐺Product- 𝐺Reactant
∆G=(l𝜇L+ m𝜇M)-(a𝜇A+ b𝜇B)--------------------(1)
Where 𝜇L,𝜇M , 𝜇A , 𝜇B, are the chemical potential of various species in the reaction mixture at
temperature T and given by,
𝜇L= 𝜇L
o RT ln PL
𝜇M= 𝜇M
o RT ln PM -------------------------(2)
𝜇A= 𝜇A
o RT ln PA
𝜇B= μB
o RT ln PB
In the above equation 𝜇L
o,𝜇M
o,𝜇A
o,𝜇B
o are chemical potential is standard state(partial pressure
chemical potential form equation (2) in equation (1) we get,
∆G=[l(𝜇L
o RT ln PL) + m(𝜇M
o RT ln PM )]-[a( 𝜇A
o RT ln PA )+b( 𝜇B
o RT ln PB)
On rearranging](https://image.slidesharecdn.com/thermodynamicppt-210218094702/85/Thermodynamic-ppt-32-320.jpg)
![VAN’T HAFF REACTION ISOTHERM
∆G=[l 𝜇L
o+ 𝜇M
o)-(a𝜇A
ob 𝜇B
o)]+RT [l ln PL+m ln PM)-(a ln PA+ b ln PB)
Or ∆Go+RT[ln (PL)lx (PM)m]-[ln(PA)ax(PB)b
∆Go+RT ln
𝑃𝐿 𝑙
(𝑃𝐴𝑎)
× 𝑃𝑀 𝑚
× 𝑃𝐵 𝑏 =reaction quotient--------------(3)
Thus,Vant’s Hoff reaction isotherms gives free energy change (∆G) of a chemical reaction in terms of
standard free (∆Go) and the partial pressure of reactants and products at a given temperature(T).
∆Go+RT ln
𝑃𝐿 𝑙
(𝑃𝐴𝑎)
× 𝑃𝑀 𝑚
× 𝑃𝐵 𝑏 equilibrium
Or
∆Go=-RT ln
𝑃𝐿 𝑙
(𝑃𝐴𝑎)
× 𝑃𝑀 𝑚
× 𝑃𝐵 𝑏 equilibrium----------------------(4)](https://image.slidesharecdn.com/thermodynamicppt-210218094702/85/Thermodynamic-ppt-33-320.jpg)














![Second equilibrium is
nX in solvent B Xn is solvent B
∴ form law of Mass action
Kc=
[𝑋𝑛]
[𝑋]𝑛 =
[𝐶2]
[𝐶3]𝑛
[C3]n=
𝐶2
𝐾𝑐
Taking nth root we get,
C3=
𝑛
𝐶2
𝑛
𝐾𝑐
--------------(2)
From equation (1) and (2) we get,
𝐶1
𝐾1
=
𝑛
𝐶2
𝑛
𝐾𝑐
∴
𝐶1
𝑛
𝐶2
=
𝐾1
𝑛
𝐾𝑐
=KD (Constant)
Thus when association occurs in one solvent the distribution equation is modified as;
𝐶1
𝑛
𝐶2
=KD](https://image.slidesharecdn.com/thermodynamicppt-210218094702/85/Thermodynamic-ppt-48-320.jpg)