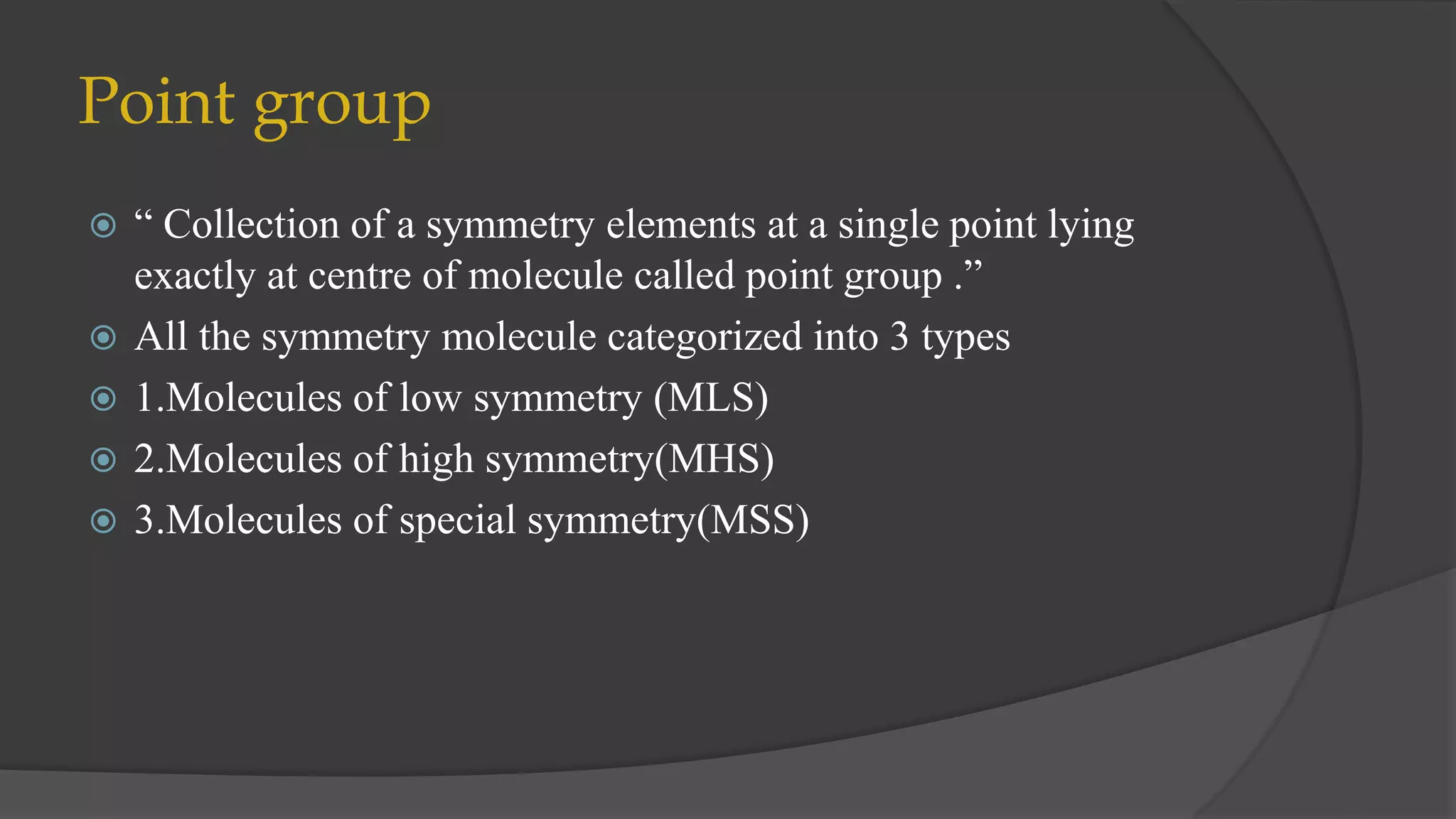
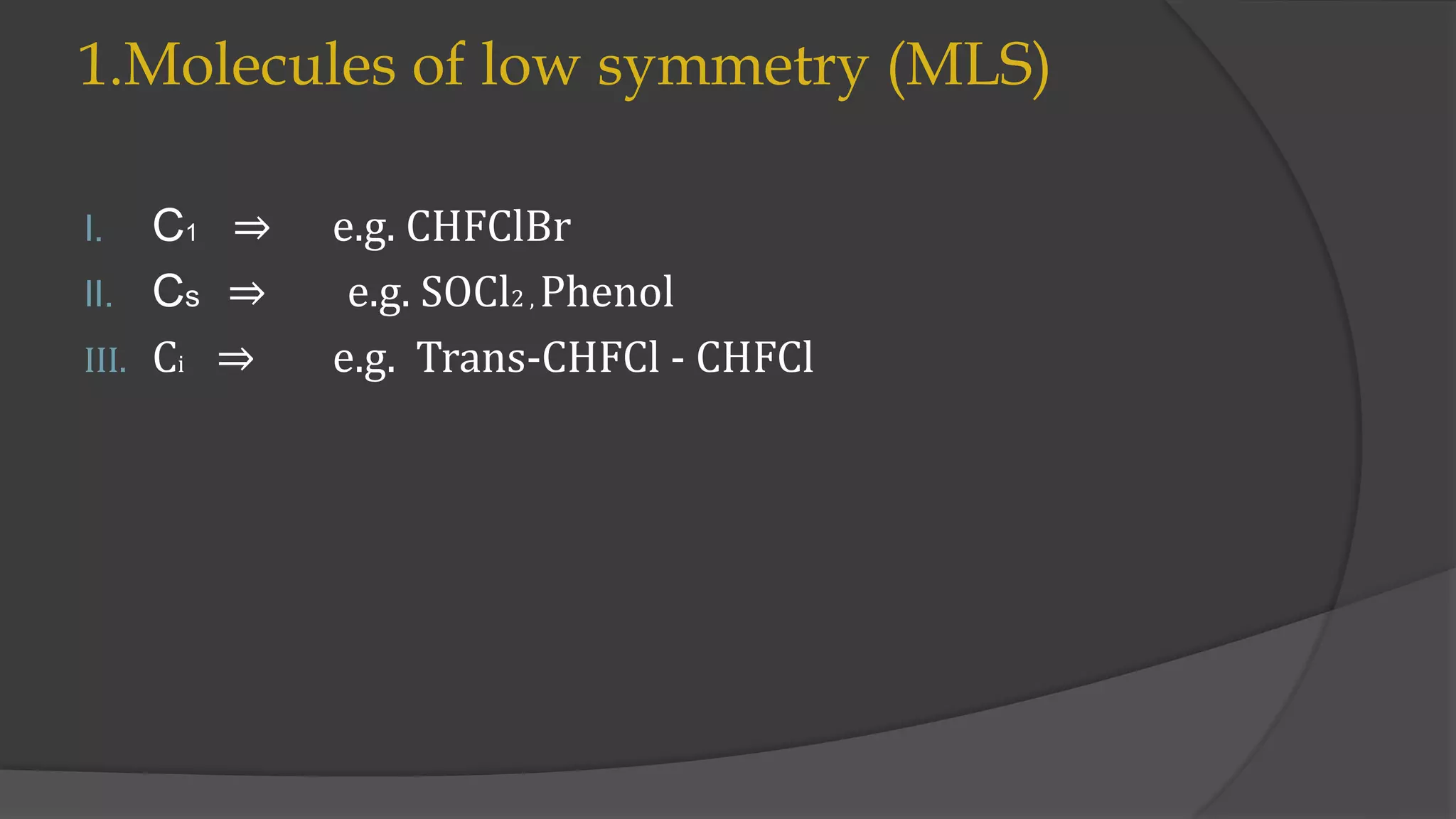
This document summarizes a seminar on group multiplication tables and abelian/non-abelian point groups. It defines groups, subgroups, symmetry operations, and point groups. It provides examples of group multiplication tables for C2v and C3v point groups. Abelian groups have commutative combinations while non-abelian groups like C3v do not. Low, high, and special symmetry molecule types are classified by their point groups. Examples are given throughout to illustrate key concepts.


![QUESTIONS
Explain abelian and non-abelian point group using
suitable example for each. [4 marks ] { nov’ 17 ; june
18 }
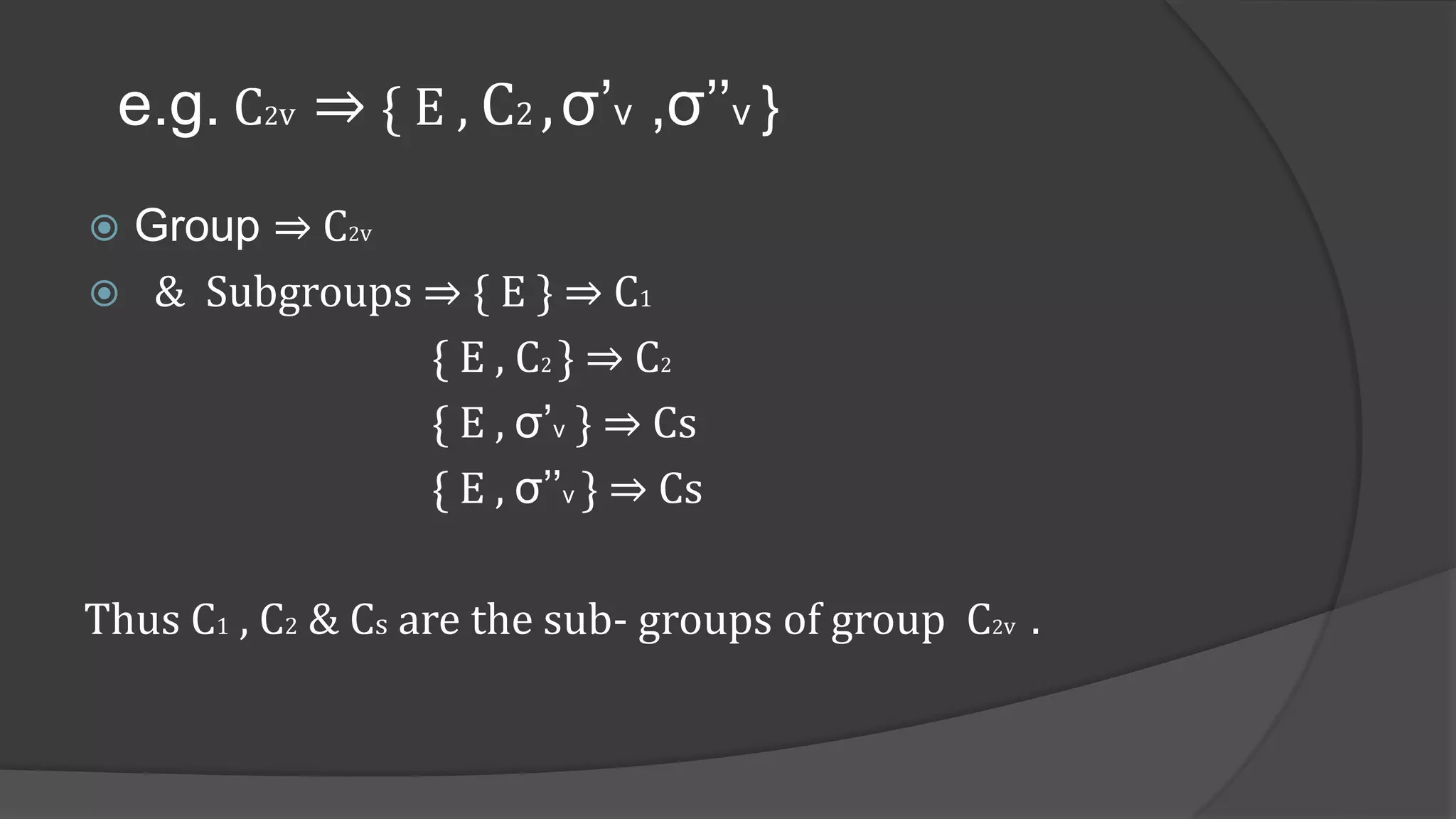
Define subgroup . Give its characteristic [ 3 marks ]
{ June 18 }](https://image.slidesharecdn.com/53-190415092858/75/group-thoery-and-character-table-3-2048.jpg)
![1.Two elements in a group (A&B) combines to form a third element called C and this
C belongs to the element of group .
A.B=C
2 An element combine itself to form the another element of group.
i.e A.A =E (exception)
A.A = B ( another element of group )
3. Group must contain identity element [E] which commute with other element
keeping them unchanged.
i.e. A.E =E.A = A
Similarly, B.E = E.B = B etc.
e.g . In H2O ; ( C2V)⇒ E.C2 = C2 =C2.E
4. Every element of group “Associative law of combinations”.
A(BC) =( AB)C
e.g . In H2O ; ( C2V)⇒ E (C2.σv) = (E.C2)σv = σ’v](https://image.slidesharecdn.com/53-190415092858/75/group-thoery-and-character-table-5-2048.jpg)