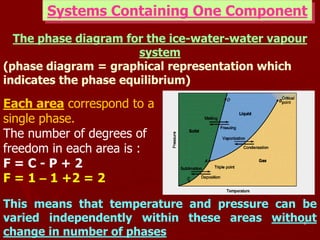
1. The phase rule describes the relationship between the number of phases, components, and degrees of freedom in a system at equilibrium. It states that the degrees of freedom F equals the number of components C minus the number of phases P plus two.
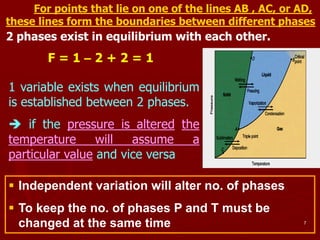
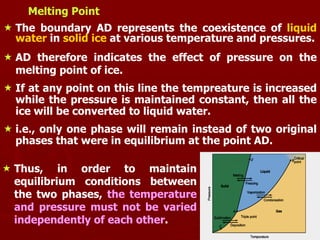
2. A phase diagram graphically represents the phase equilibria of a system. The phase diagram for ice, water, and water vapor shows three single-phase regions and three phase boundary lines where two phases coexist in equilibrium.
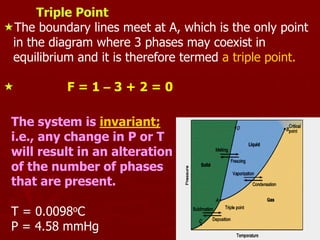
3. The triple point is the only condition where ice, water, and water vapor can coexist in equilibrium, with zero degrees of freedom. It occurs at a temperature of 0.01 degrees C and a pressure of 4.






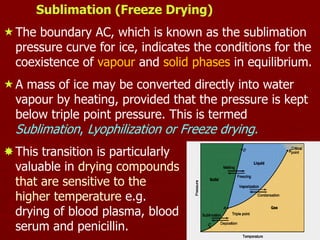

![13
Study Questions
Define the following terms:
[homogeneous, heterogeneous, phase diagram, sublimation, Lyophilization, melting point, boiling point,
triple point, etc]
Respond to the following questions:
Illustrate the process of sublimation in a pharmaceutical system
Group work discussional questions:
Discuss the variations in a solution that may constitute the pharmaceutical material phase
changes equilibrium
What are the main key points to consider when a pharmaceutical system is undergoing phase
changes within a system.](https://image.slidesharecdn.com/4-phaseequilibrium-180825211806/85/Phase-Equilibrium-13-320.jpg)