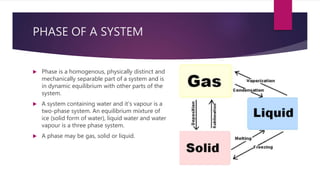
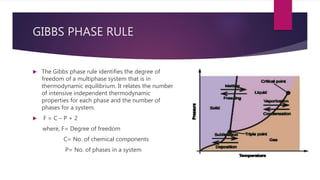
This document discusses the phase rule and its applications. It defines key concepts like phases of a system, components, and degrees of freedom. It then explains Gibbs phase rule, which relates the number of phases, components and degrees of freedom in a system. The phase rule is applied to determine equilibrium conditions. It has advantages like being applicable to both physical and chemical equilibria. Limitations include only applying to systems in equilibrium and not providing information about other possible equilibria.