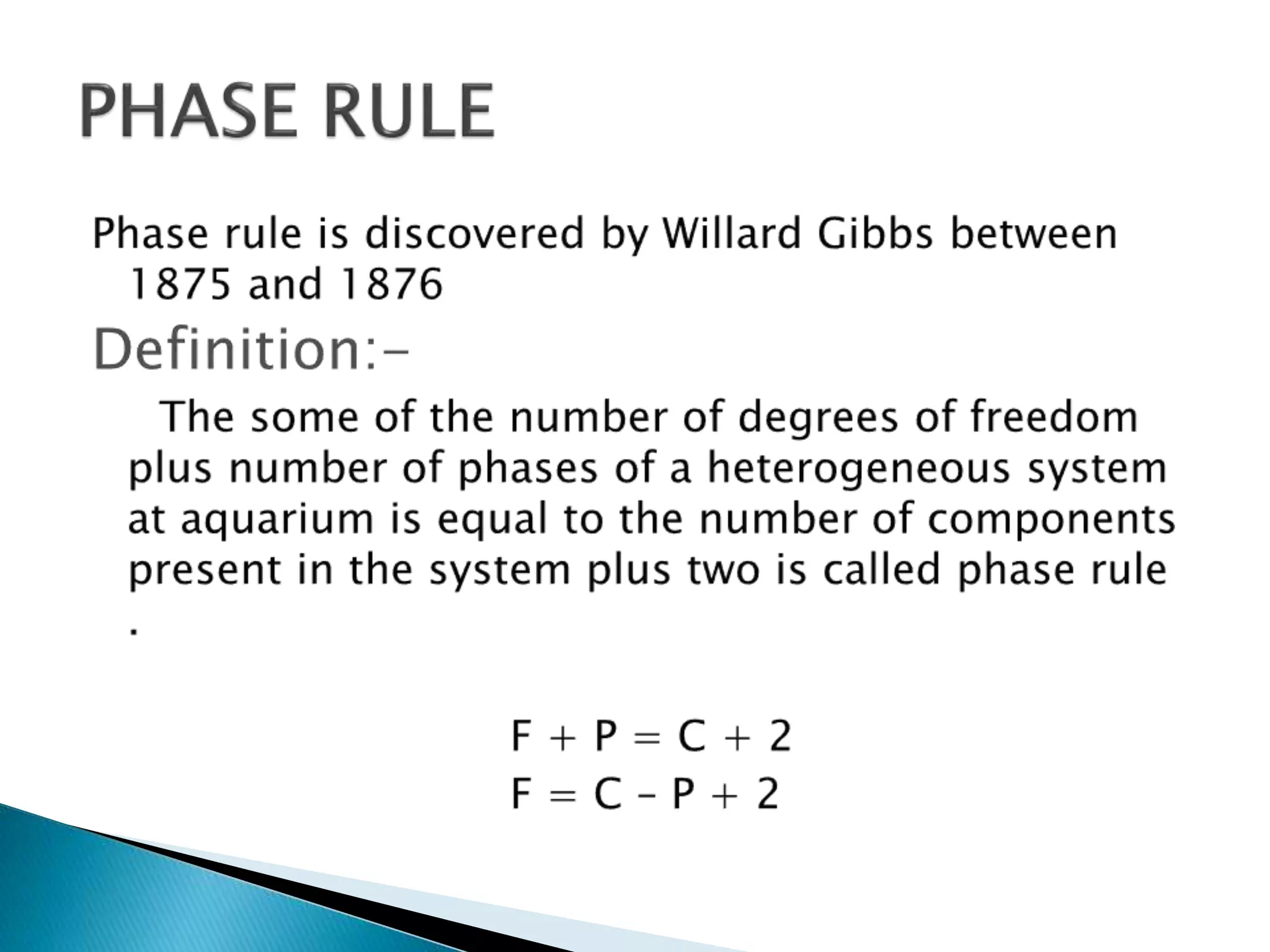
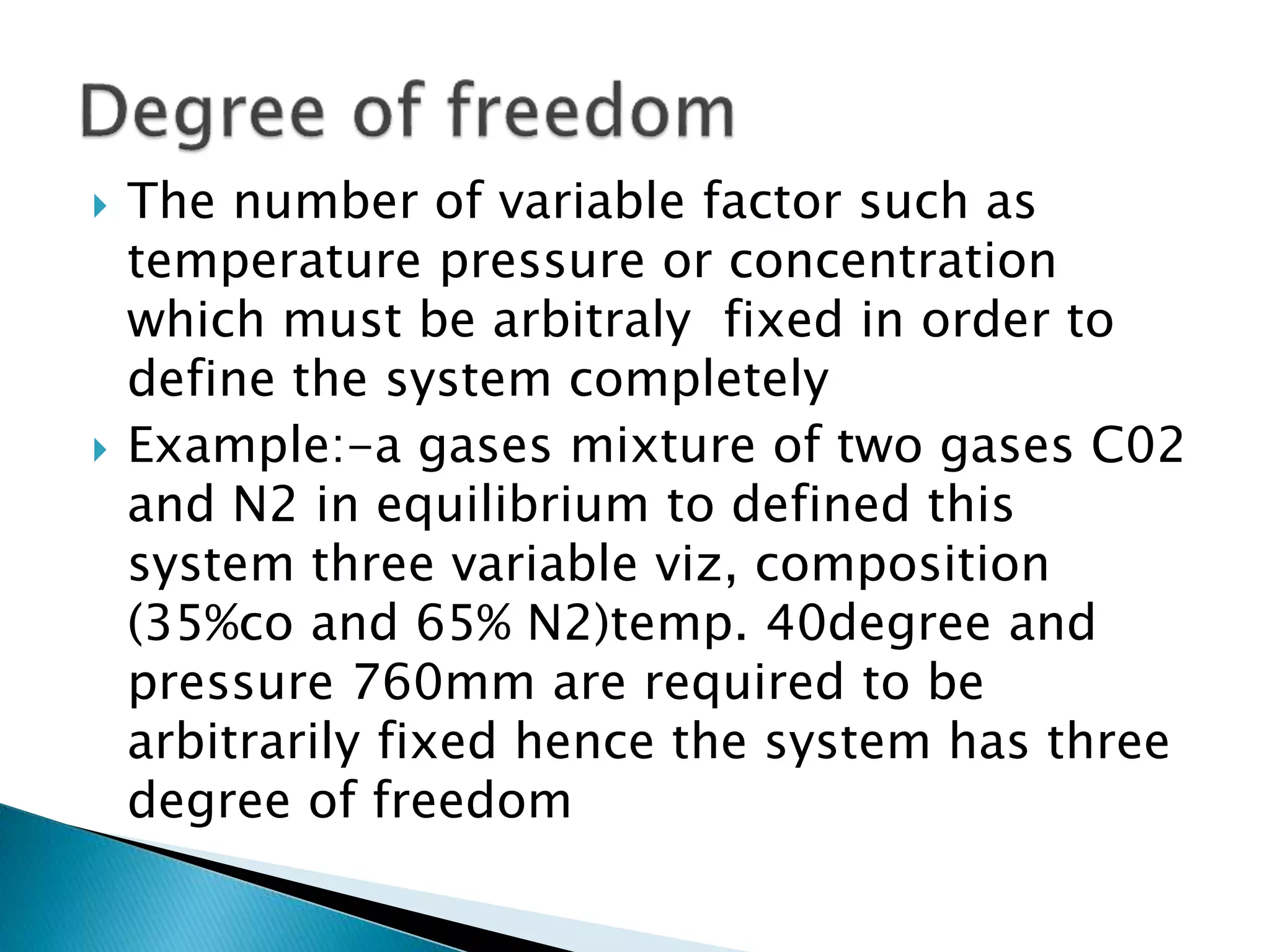
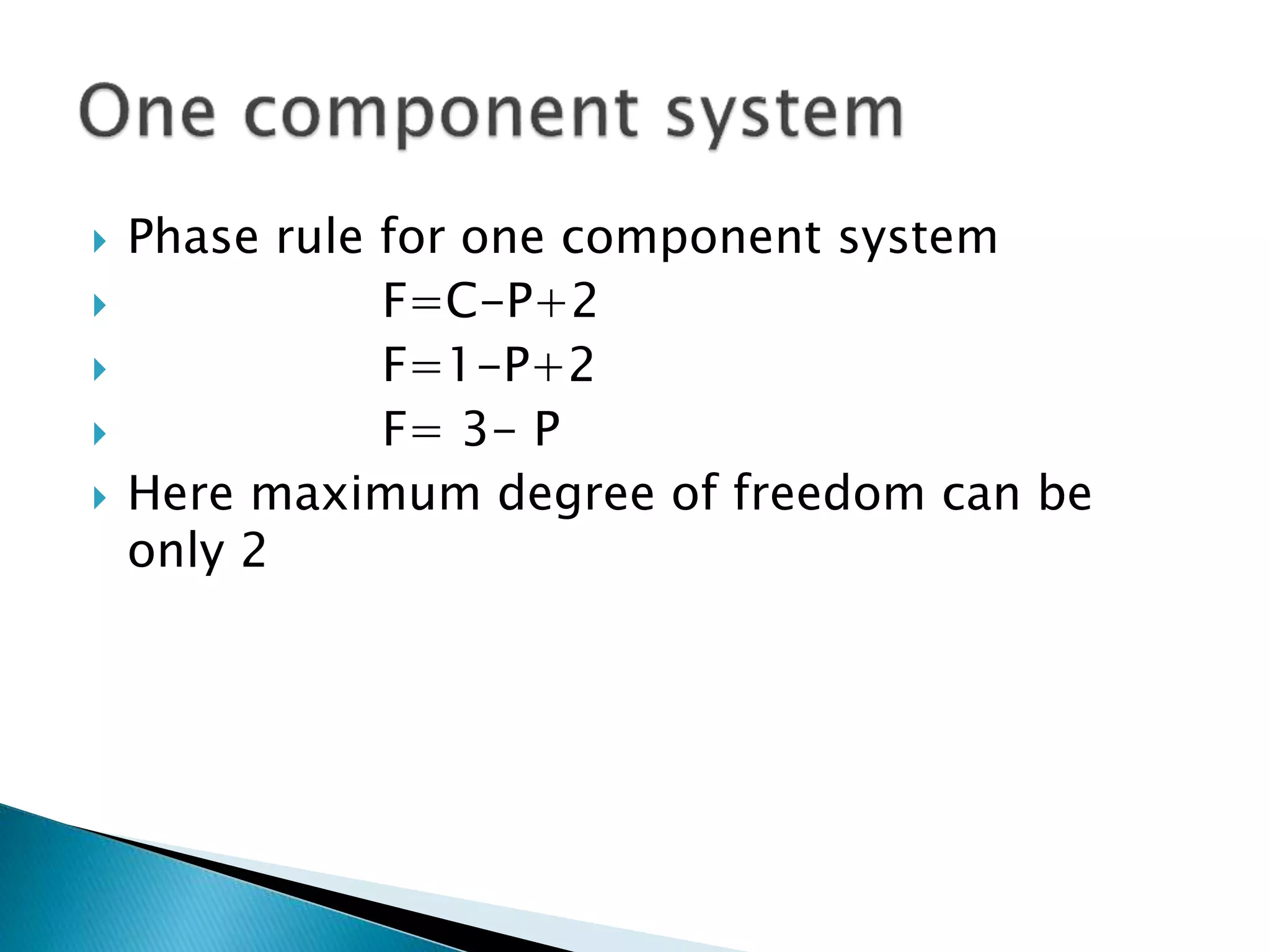
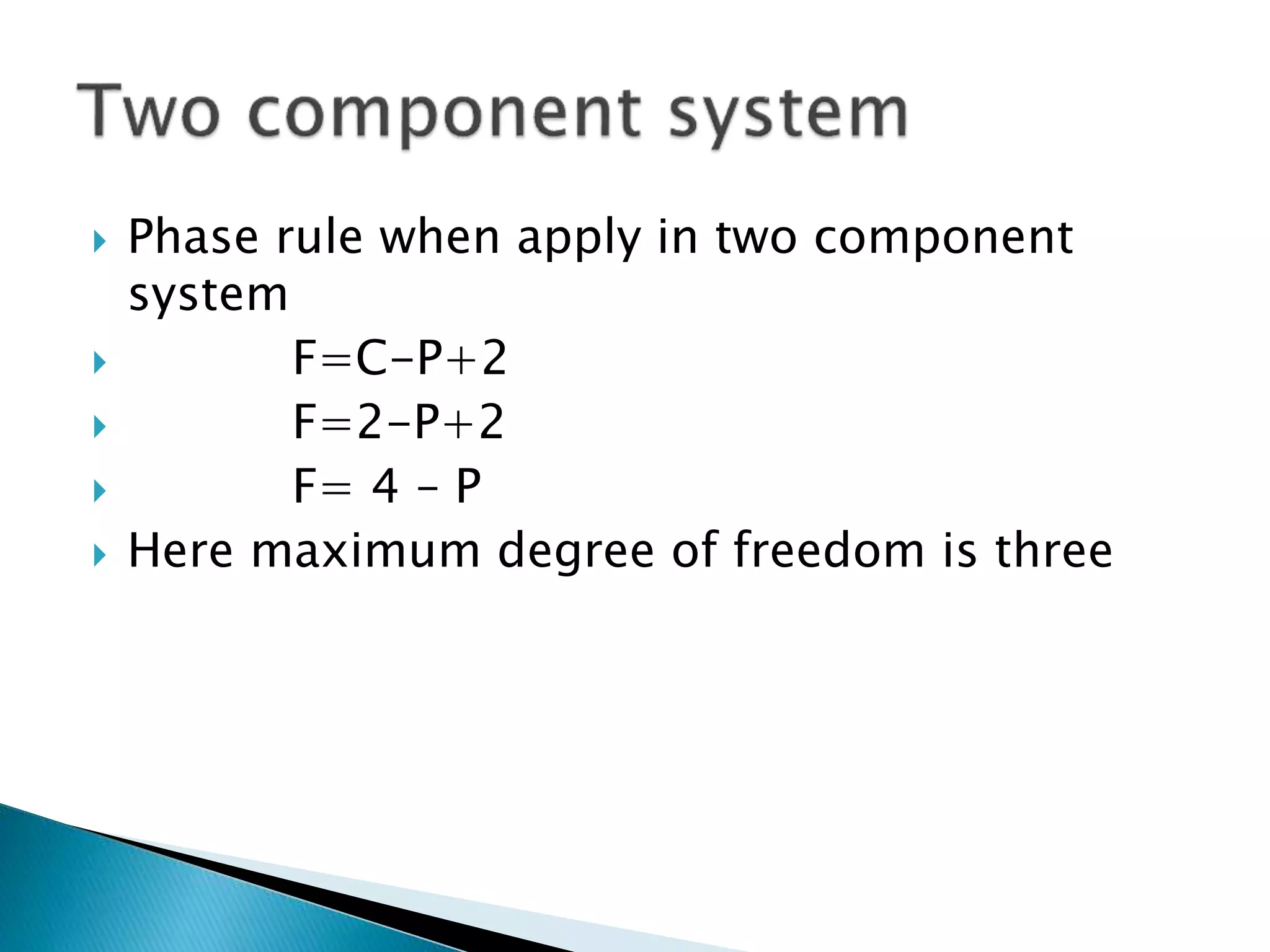
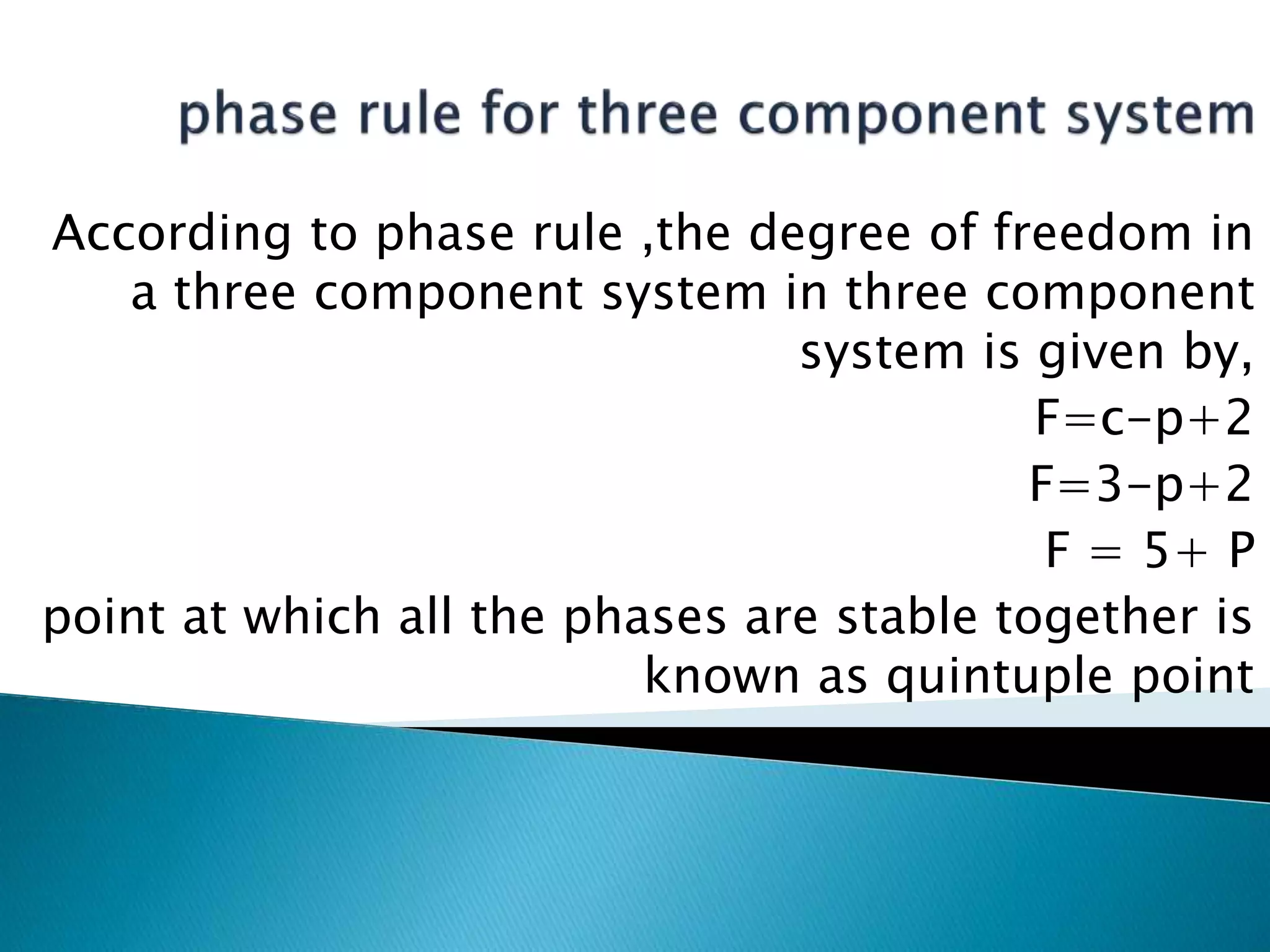
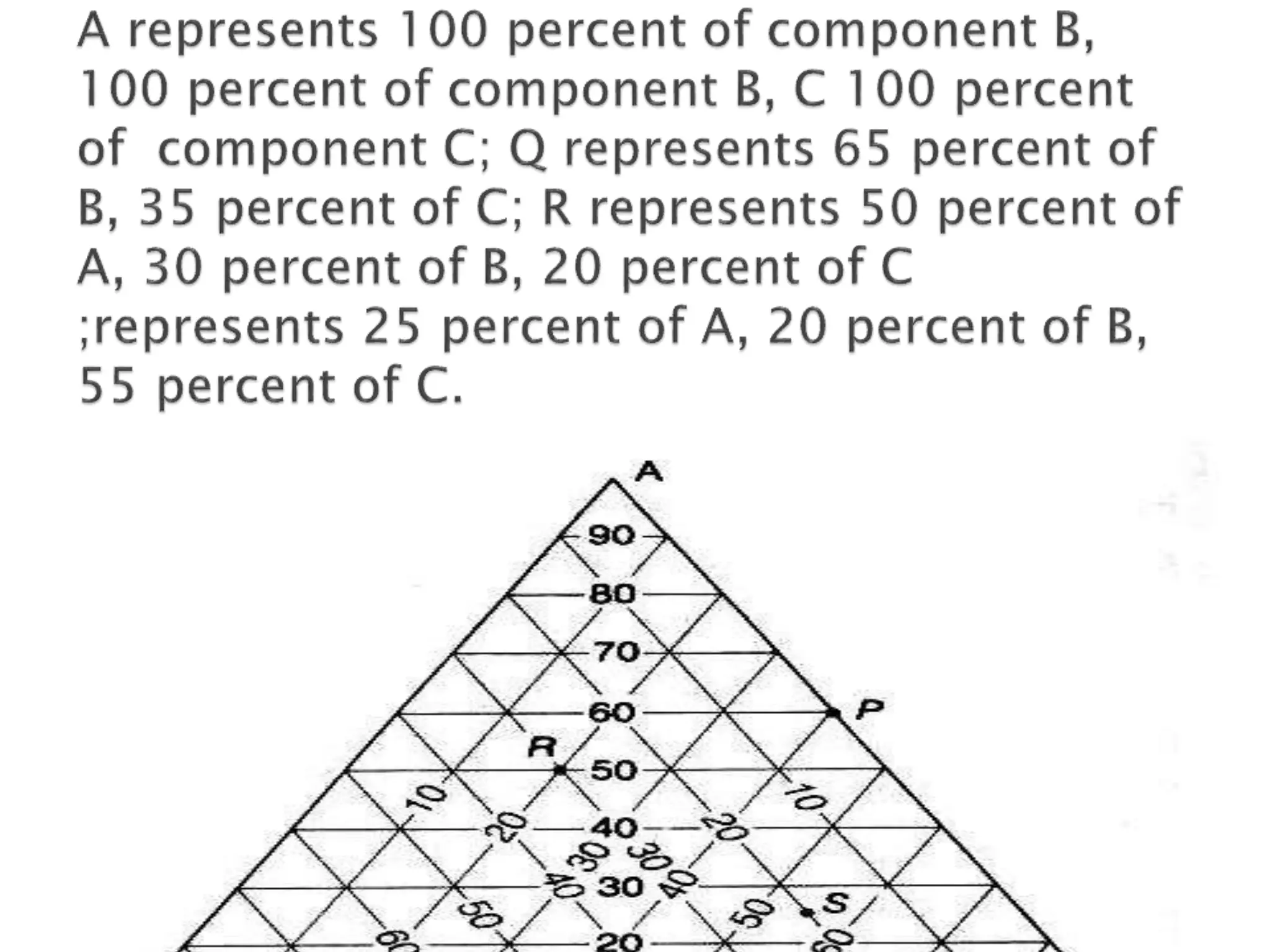
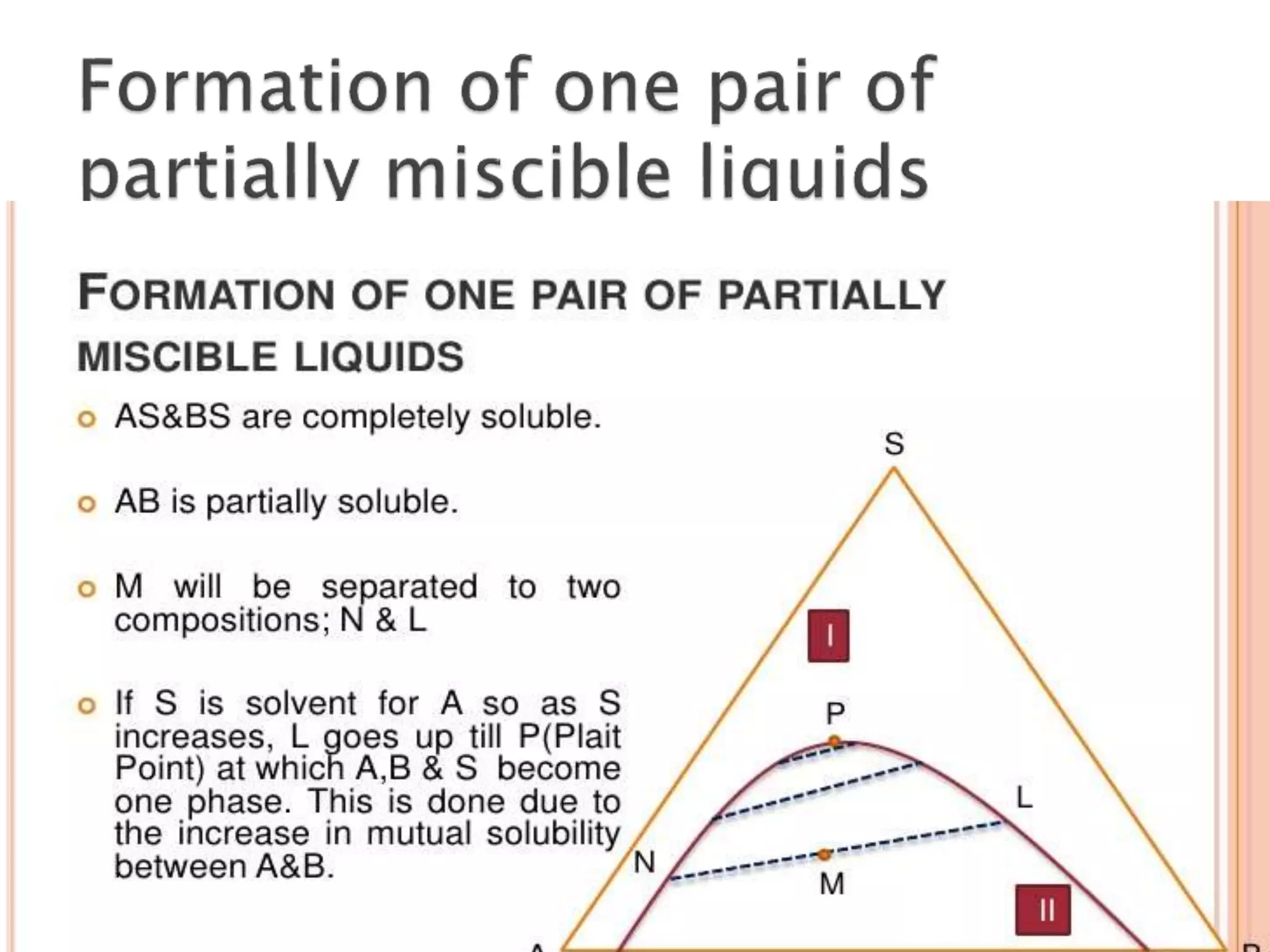
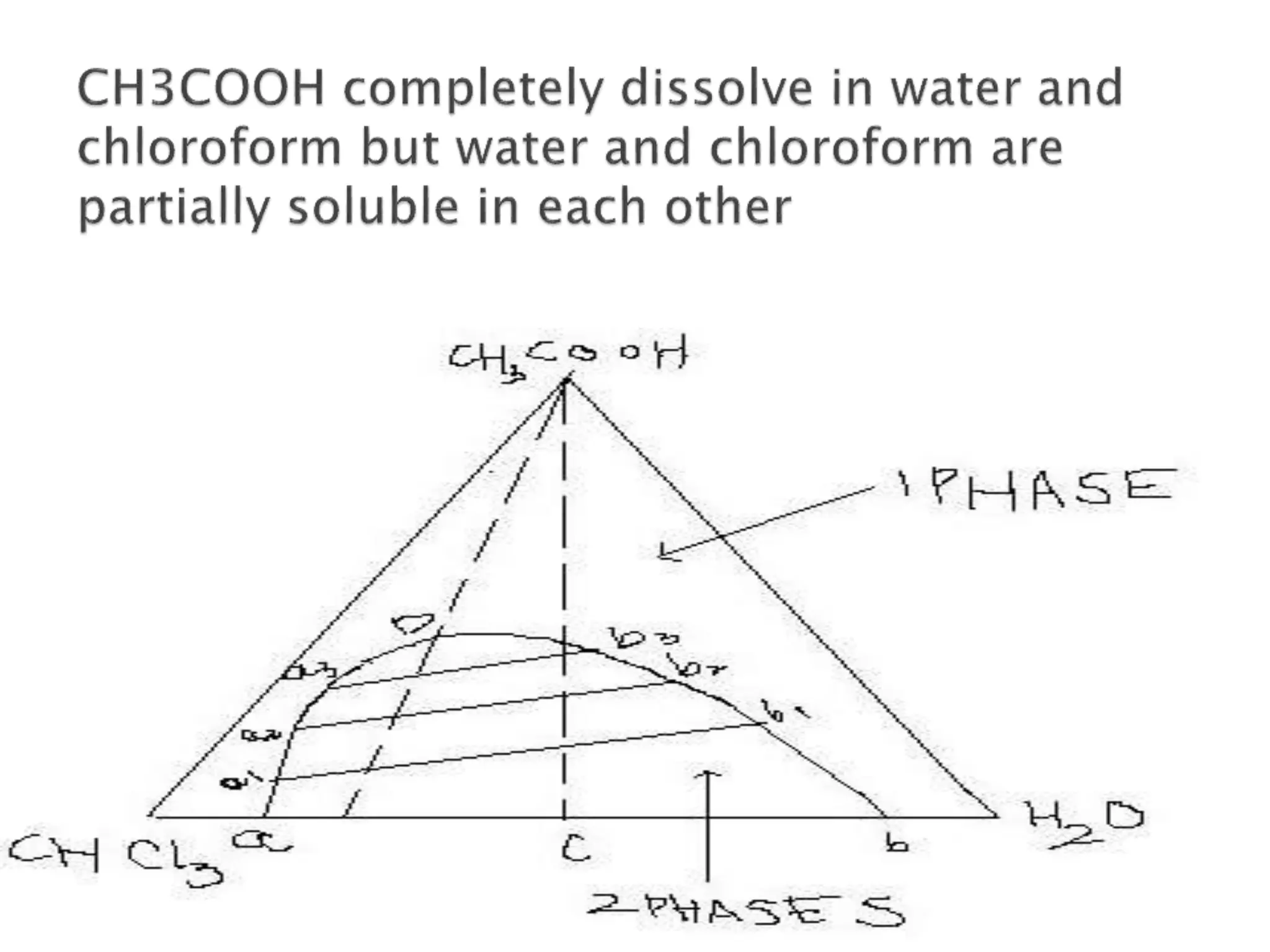
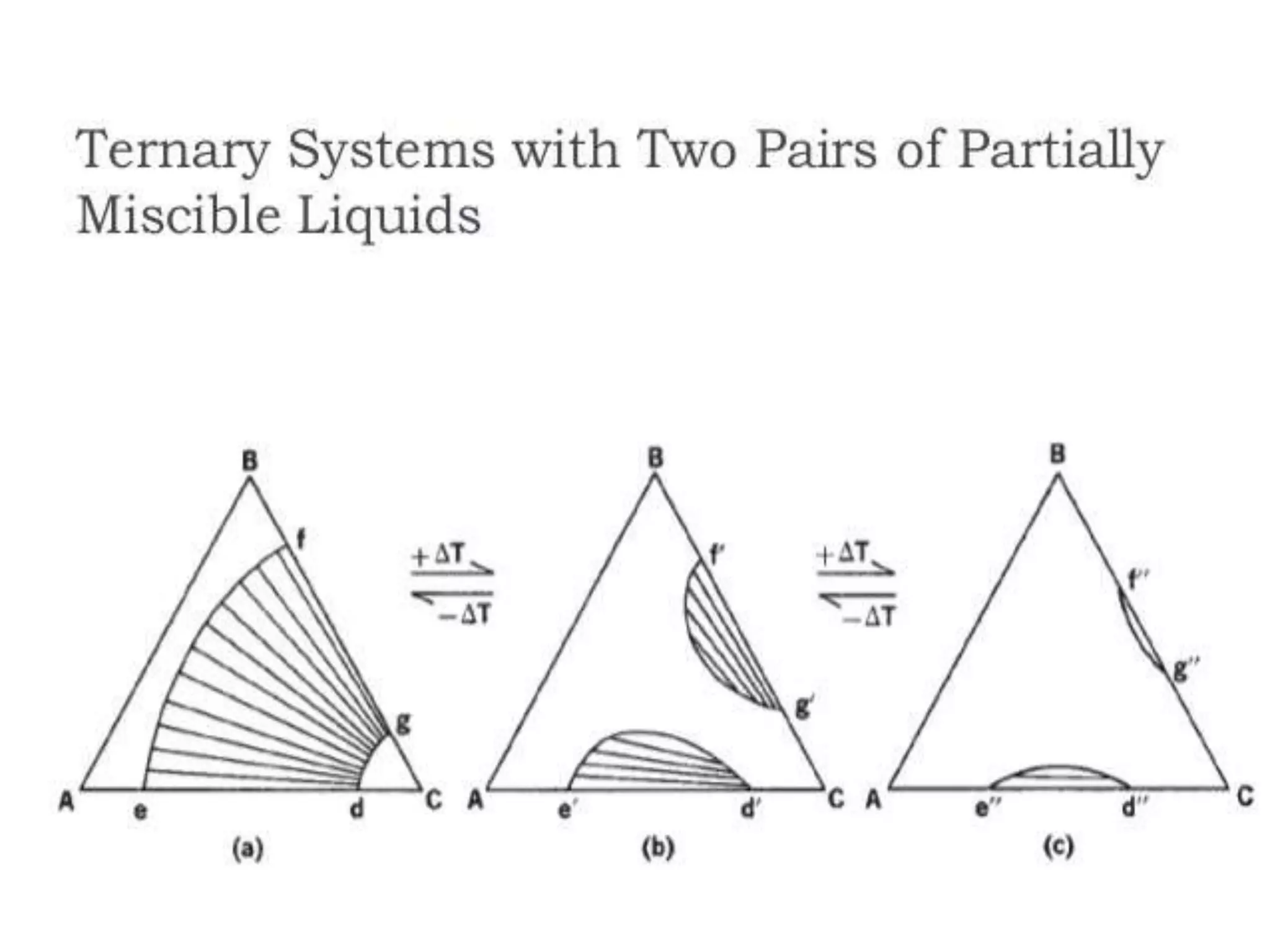
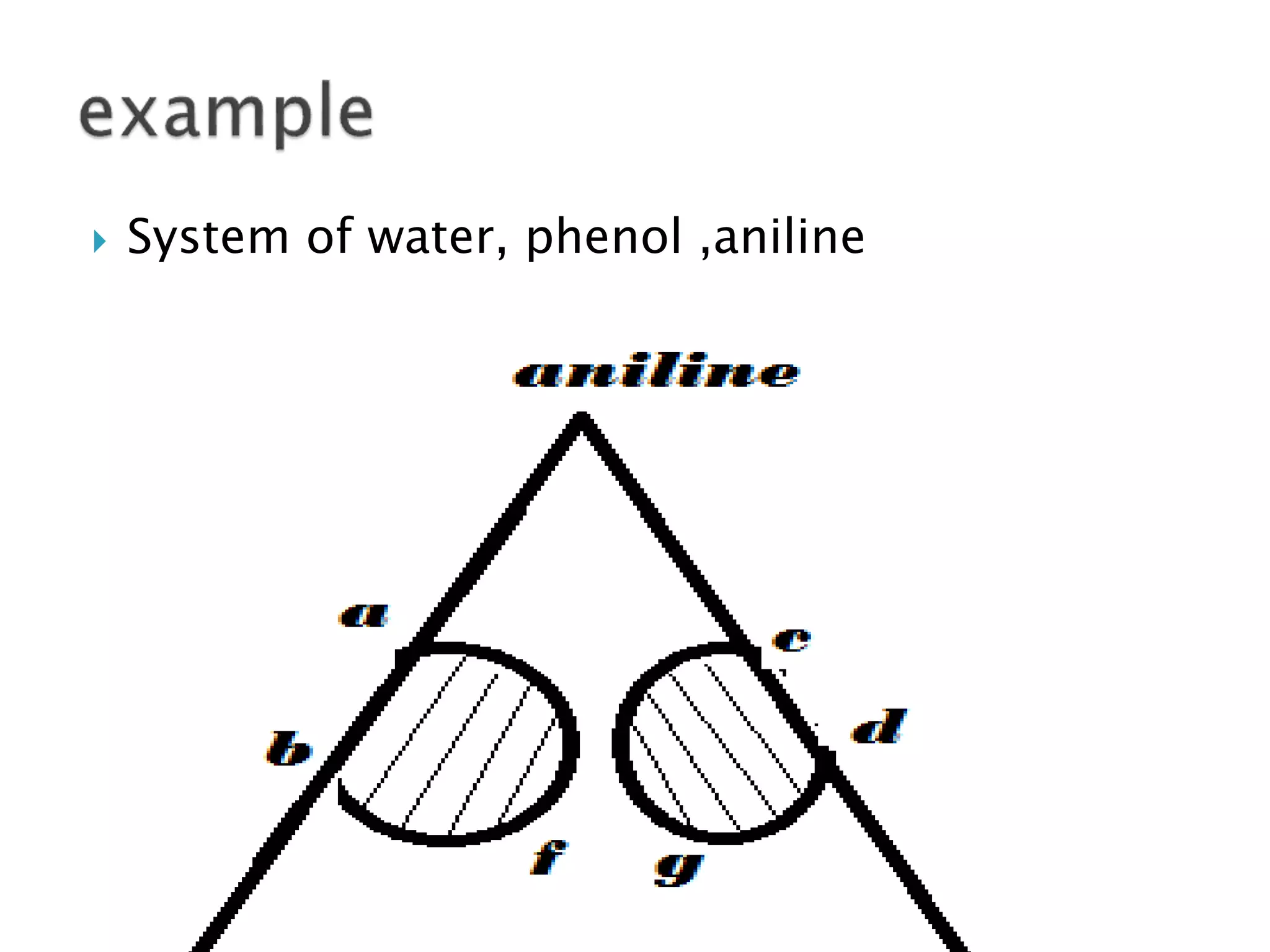
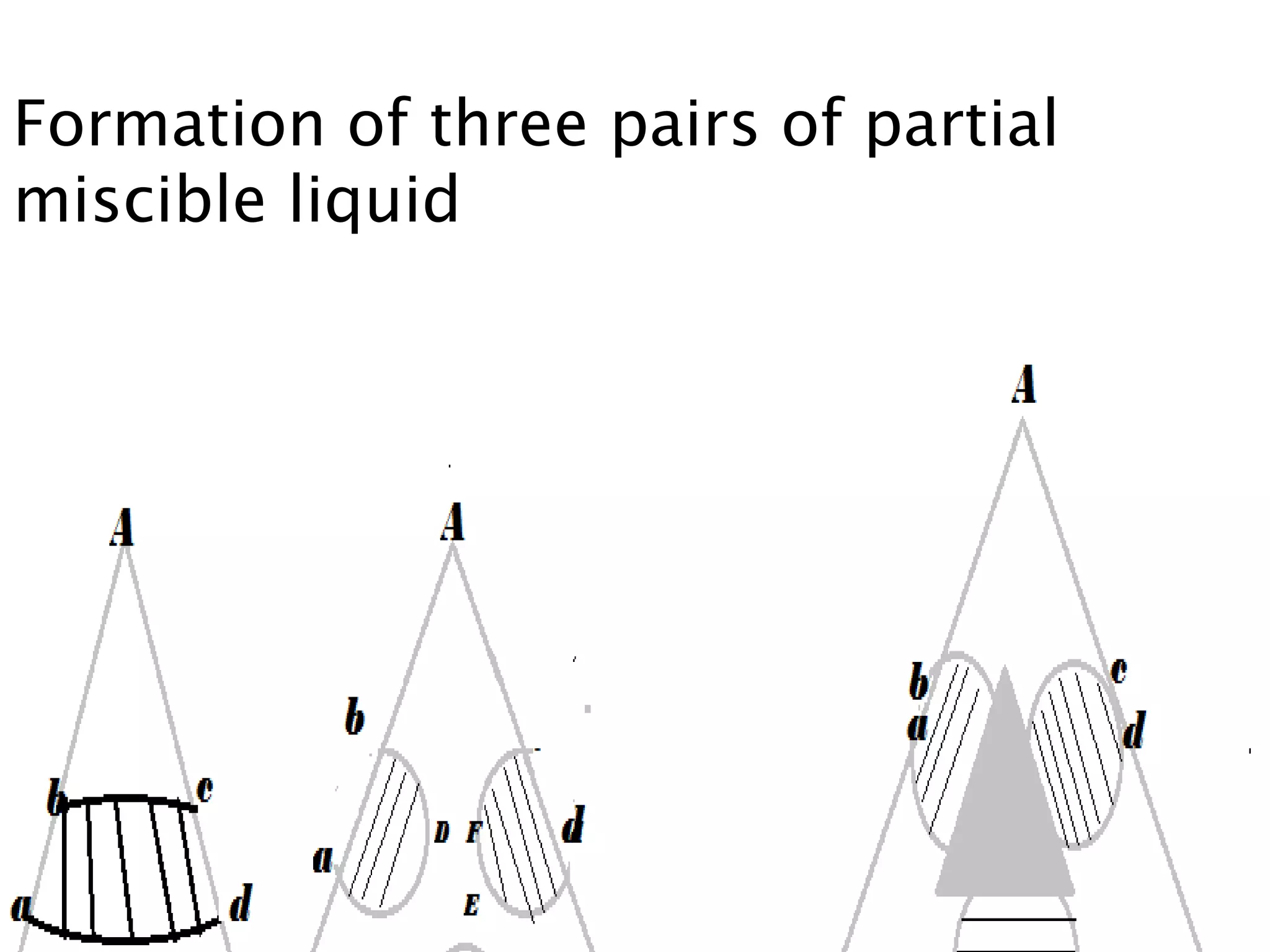
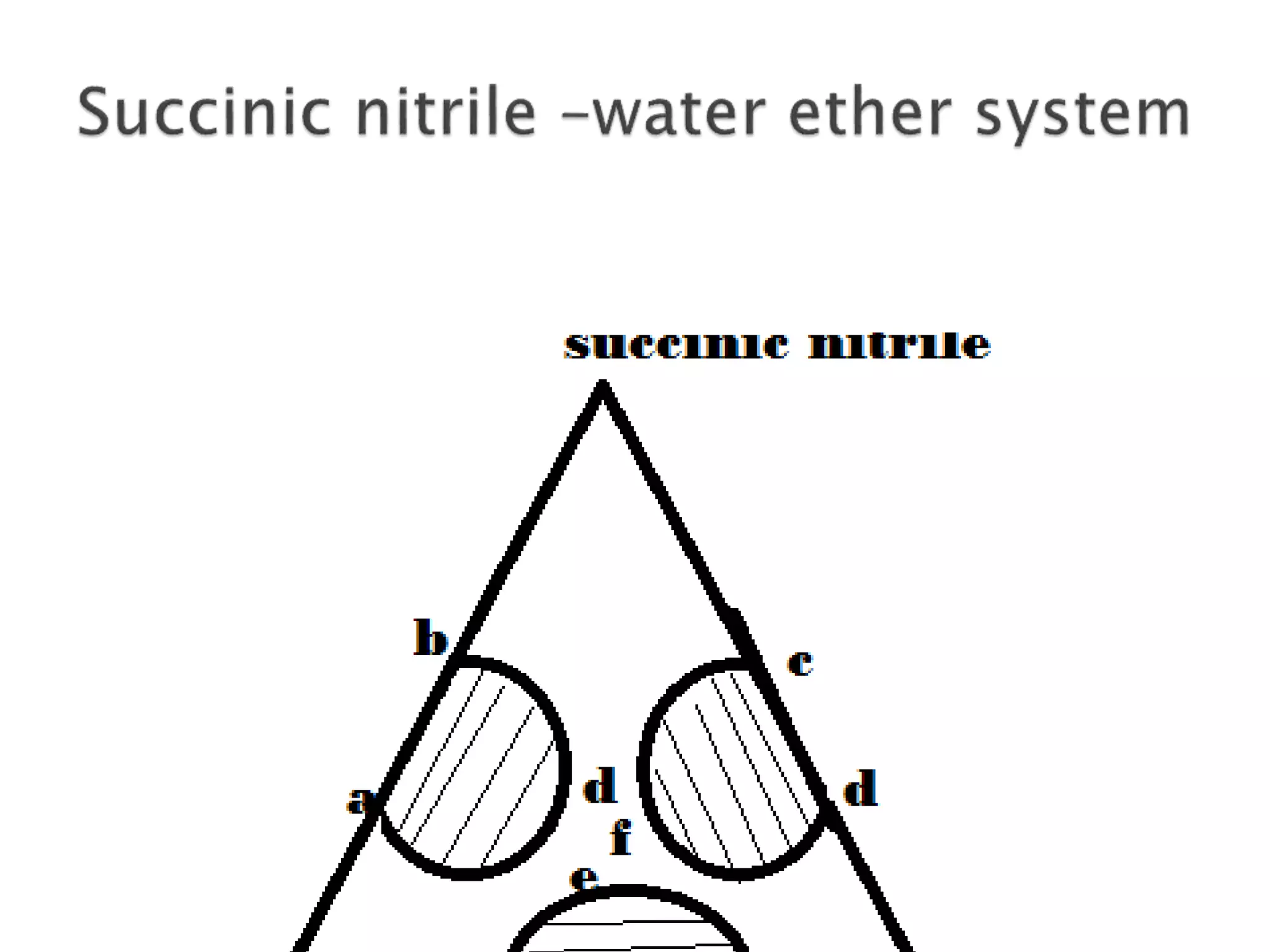
This document discusses phase diagrams and the phase rule. It defines key terms like phase, component, and degree of freedom. It then explains how to apply the phase rule to one, two, and three component systems. For a three component system, the degree of freedom is given by F=C-P+2 or F=5-P. The document also discusses types of three component phase diagrams, including ones that show the formation of one, two, or three pairs of partially miscible liquids. An example system of water, phenol, and aniline is given that forms three pairs of partially miscible liquids.