This presentation discusses the concept of fugacity, which is a measure of a gas's tendency to escape or expand. Fugacity (f) is the effective pressure of a real gas and is related to the ideal gas pressure (P) by the fugacity coefficient (φ). The fugacity coefficient depends on temperature, pressure, and gas properties. Fugacity is important in studying phase and chemical equilibria involving gases at high pressures. The standard state of fugacity relates the molar free energy of real and ideal gases. The temperature and pressure dependence of fugacity are also derived.











![EFFECT OF TEMPERATURE
ON FUGACITY
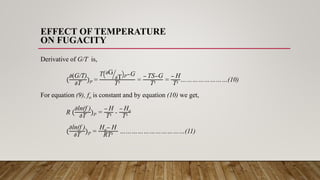
From equation (7) we get,
G/T – Go/T = R ln(f ) - R ln(fo)
Differentiating with respect to temperature at constant pressure we get,
(
𝜕(G/T)
𝜕T
)P – (
𝜕(Go/T)
𝜕T
)P= R[(
𝜕ln(f )
𝜕T
)P – (
𝜕ln(fo)
𝜕T
)P]………(9)
We know,
dG = VdP – SdT](https://image.slidesharecdn.com/thermofugacity-191028173725/85/Fugacity-12-320.jpg)