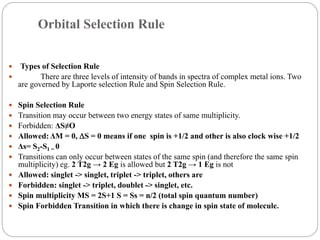
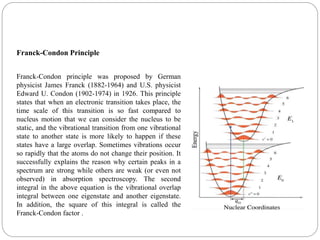
This document discusses selection rules and photochemistry concepts. It describes three main selection rules that govern transitions in complex metal ions: spin selection rules, orbital selection rules, and Laporte selection rules. Spin selection rules state that transitions can only occur between states of the same spin and multiplicity. Orbital selection rules indicate that d-d transitions are usually forbidden but can be partially allowed. Laporte selection rules are based on parity and state that transitions between states of the same parity are forbidden. The document also discusses the Franck-Condon principle and provides an abstract on photochemistry occurring on particulate matter and within liquid droplets.