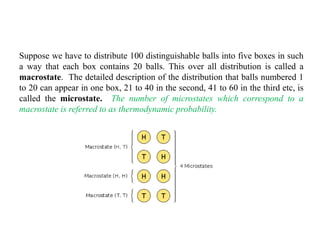
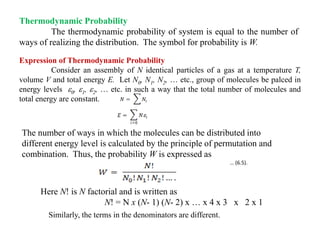
The document discusses statistical thermodynamics, emphasizing the connection between thermodynamics and quantum mechanics through statistical mechanics. It explains key concepts such as probability, partition functions, microstates, macrostates, and the distribution of particles among energy levels. Various examples illustrate how to calculate thermodynamic probabilities and configurations of particles in different scenarios.