The document discusses phase rule and its application in one and two component systems. Some key points:
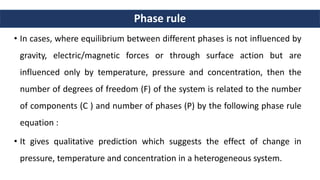
1. Phase rule relates the number of degrees of freedom (F) in a system to the number of components (C) and phases (P) using the equation F = C - P + 2.
2. In a one component system like water, the phase diagram plots pressure and temperature and shows regions where ice, water and vapor can exist.
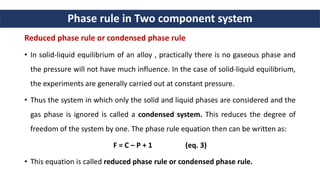
3. A two component system can form simple eutectic mixtures, compounds with congruent/incongruent melting points, or solid solutions. The reduced phase rule for constant pressure is F = C - P + 1.