1) The phase rule relates the number of degrees of freedom (F) in a heterogeneous system to the number of components (C) and phases (P) using the equation F=C-P+2.
2) A one-component system like water can exist in three phases (solid, liquid, vapor) and is represented by a two-dimensional pressure-temperature phase diagram showing the equilibrium boundaries between phases.
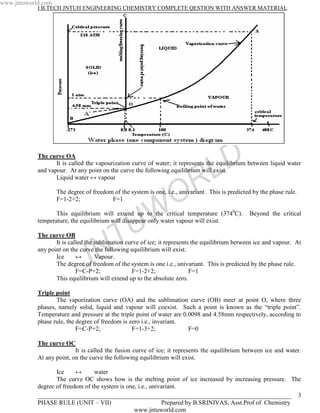
3) In the water system, the vaporization curve on the phase diagram represents the equilibrium between liquid water and water vapor, with the system having one degree of freedom (univariant) as predicted by the phase rule.