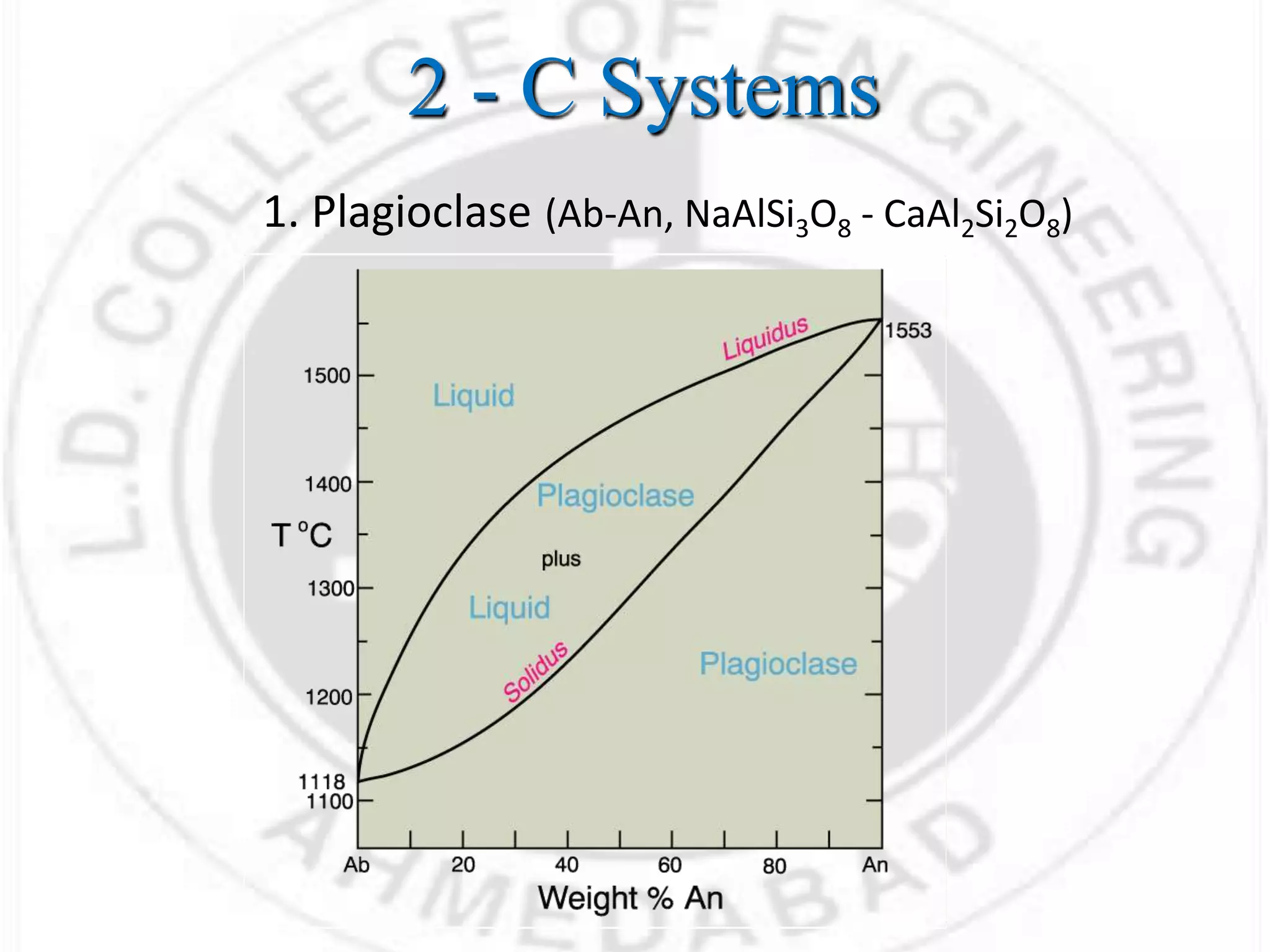
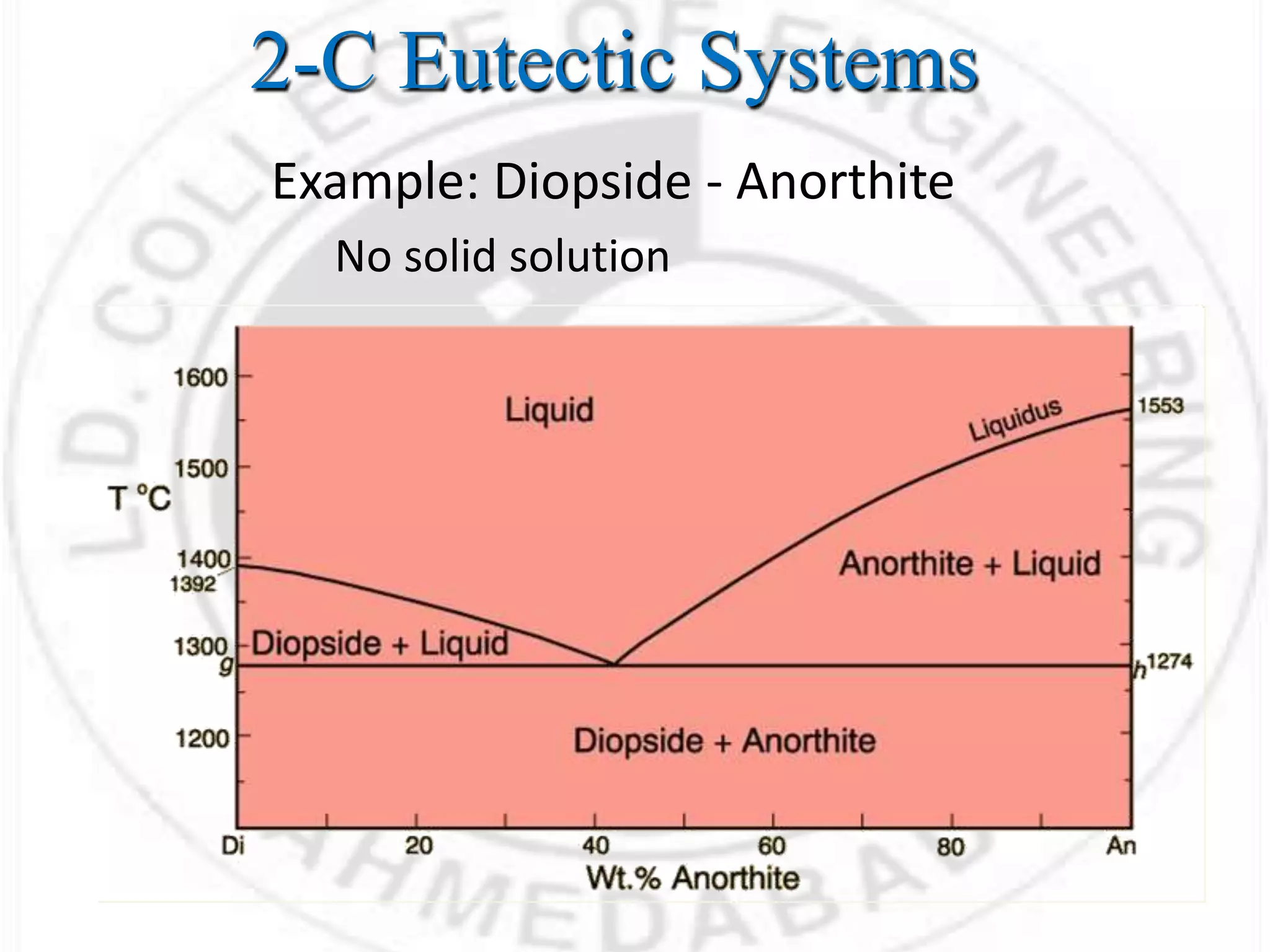
This document discusses basic concepts related to thermodynamics of elastomers and polymers, including:
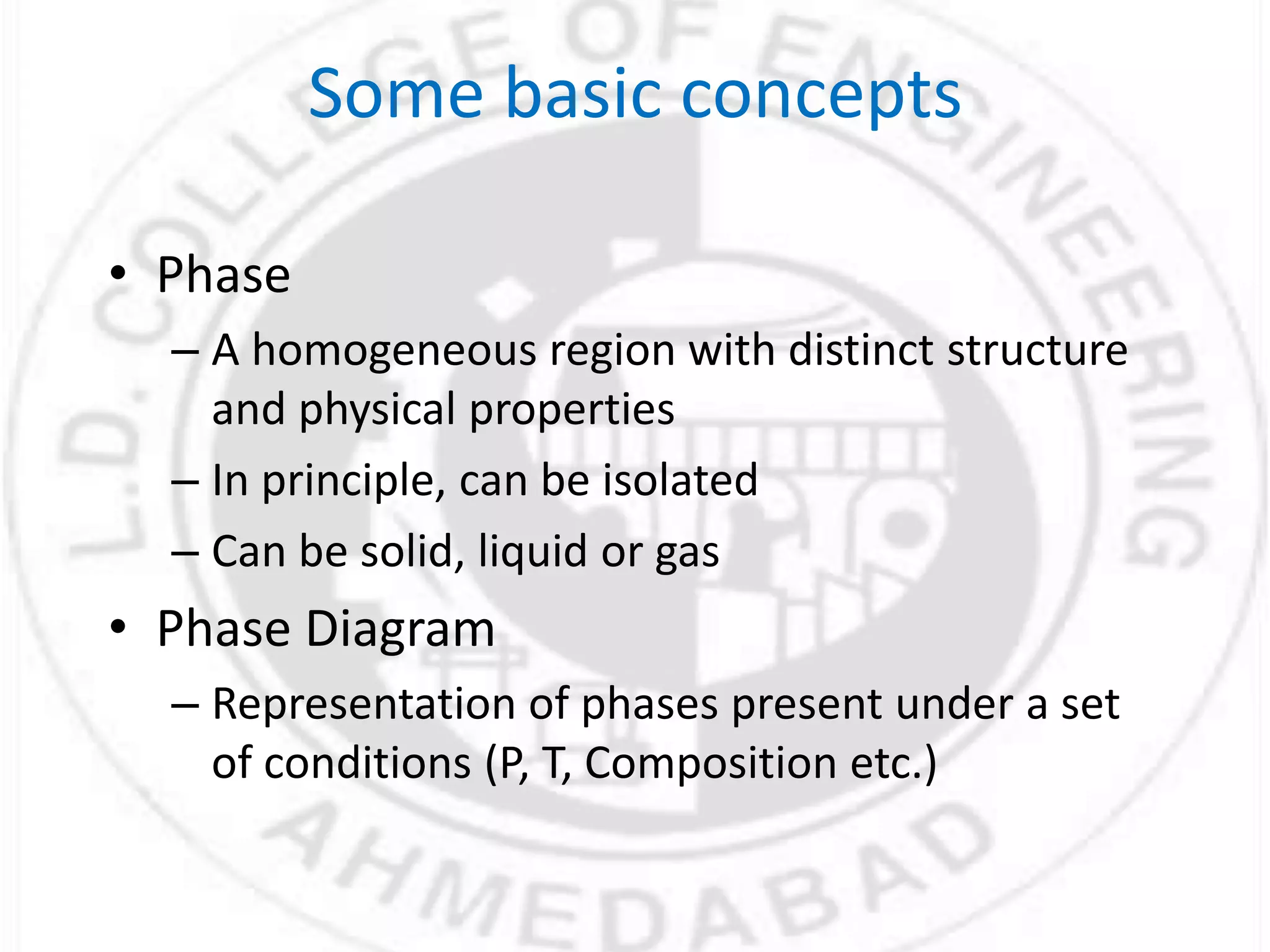
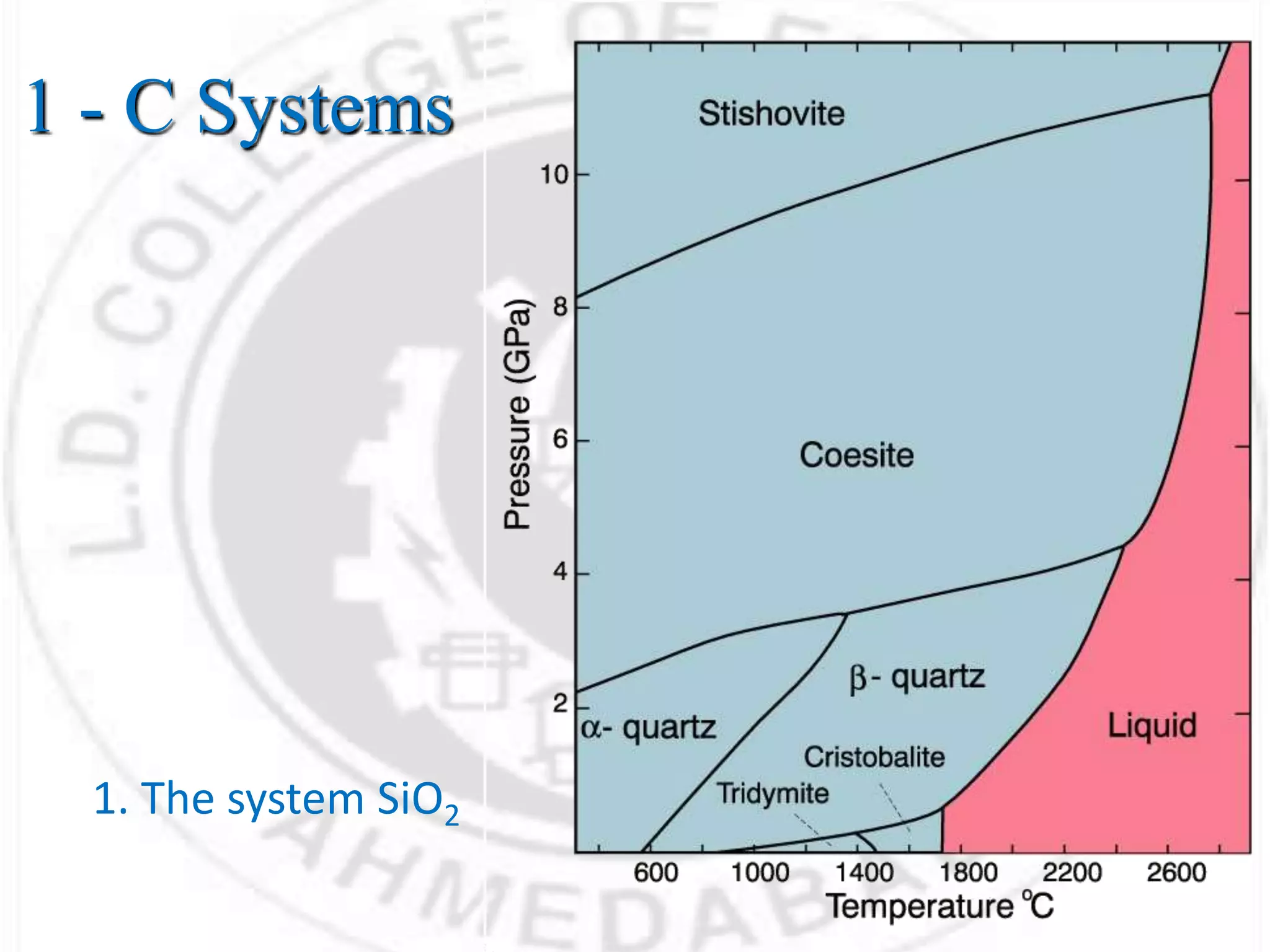
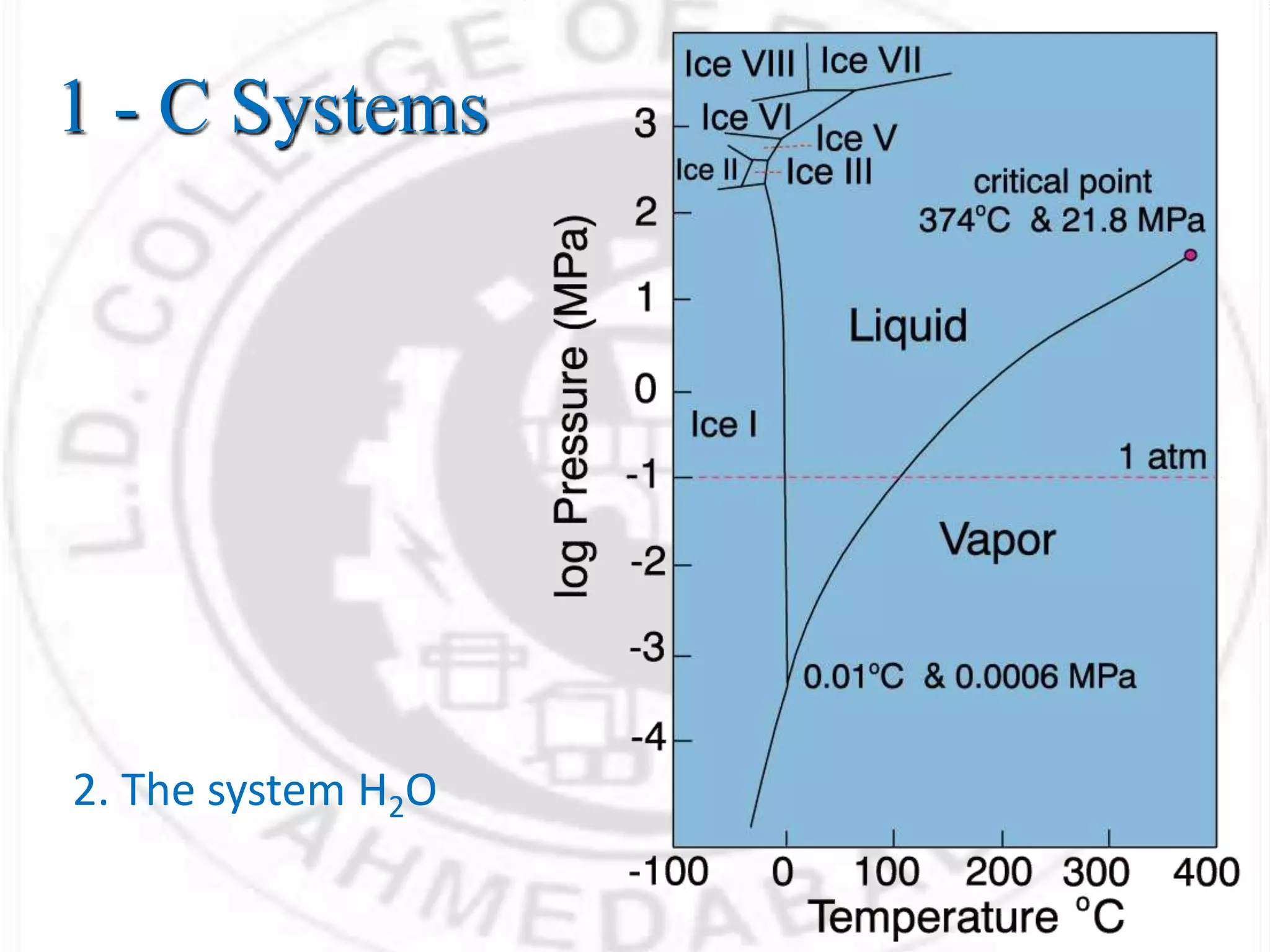
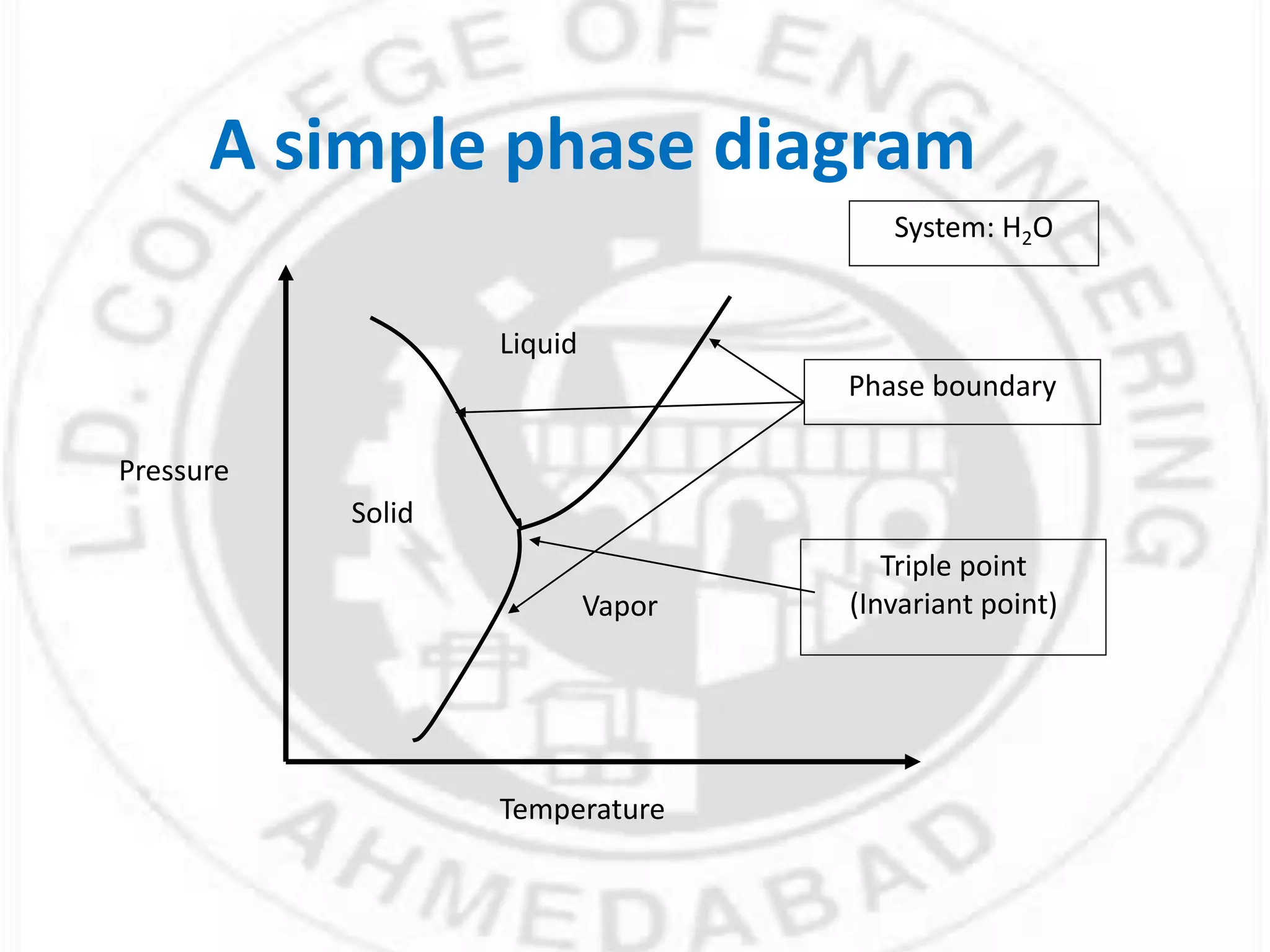
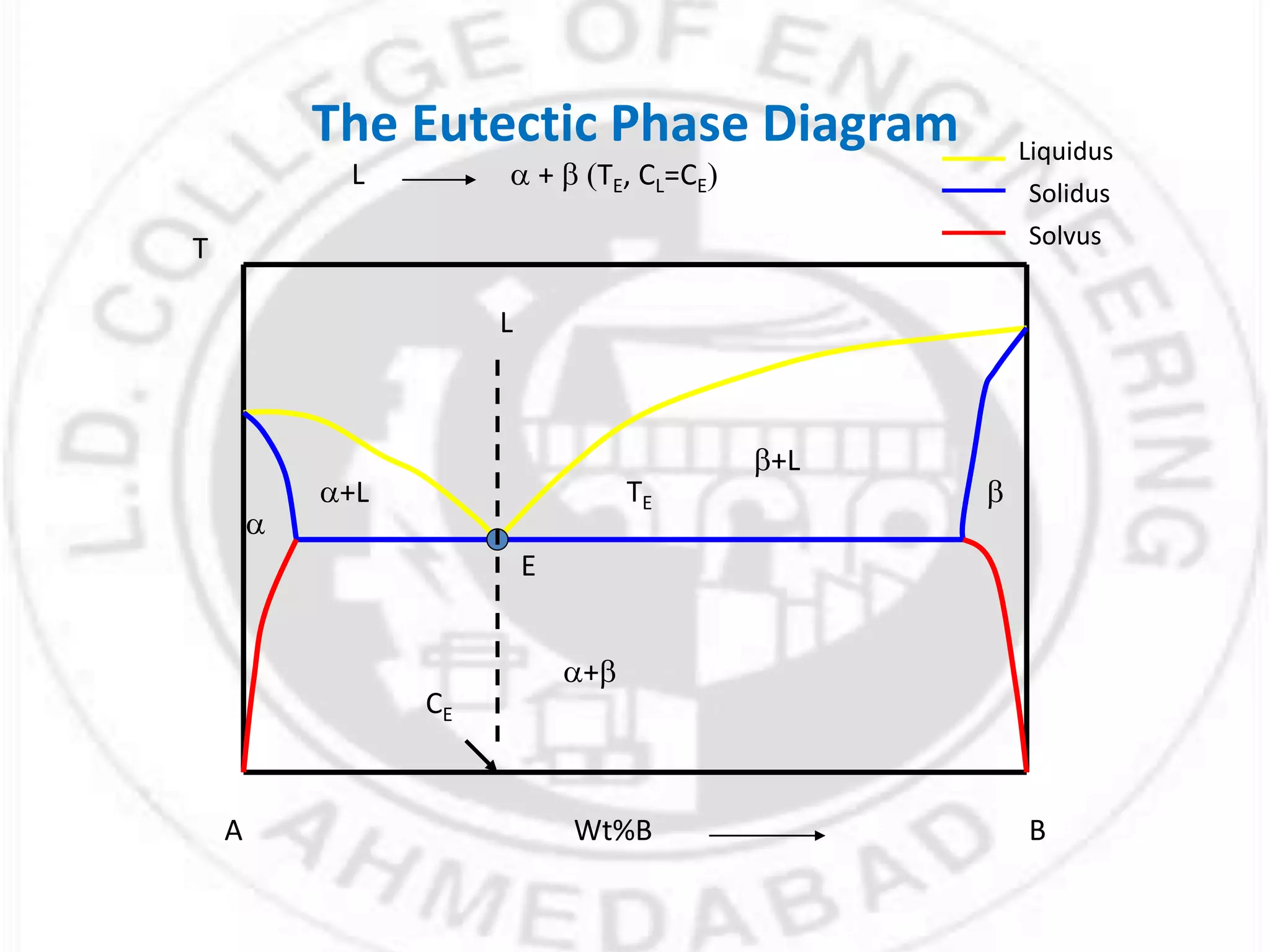
- Phases can be solid, liquid, or gas and exist in homogeneous regions with distinct properties. Phase diagrams show the phases present under different conditions.
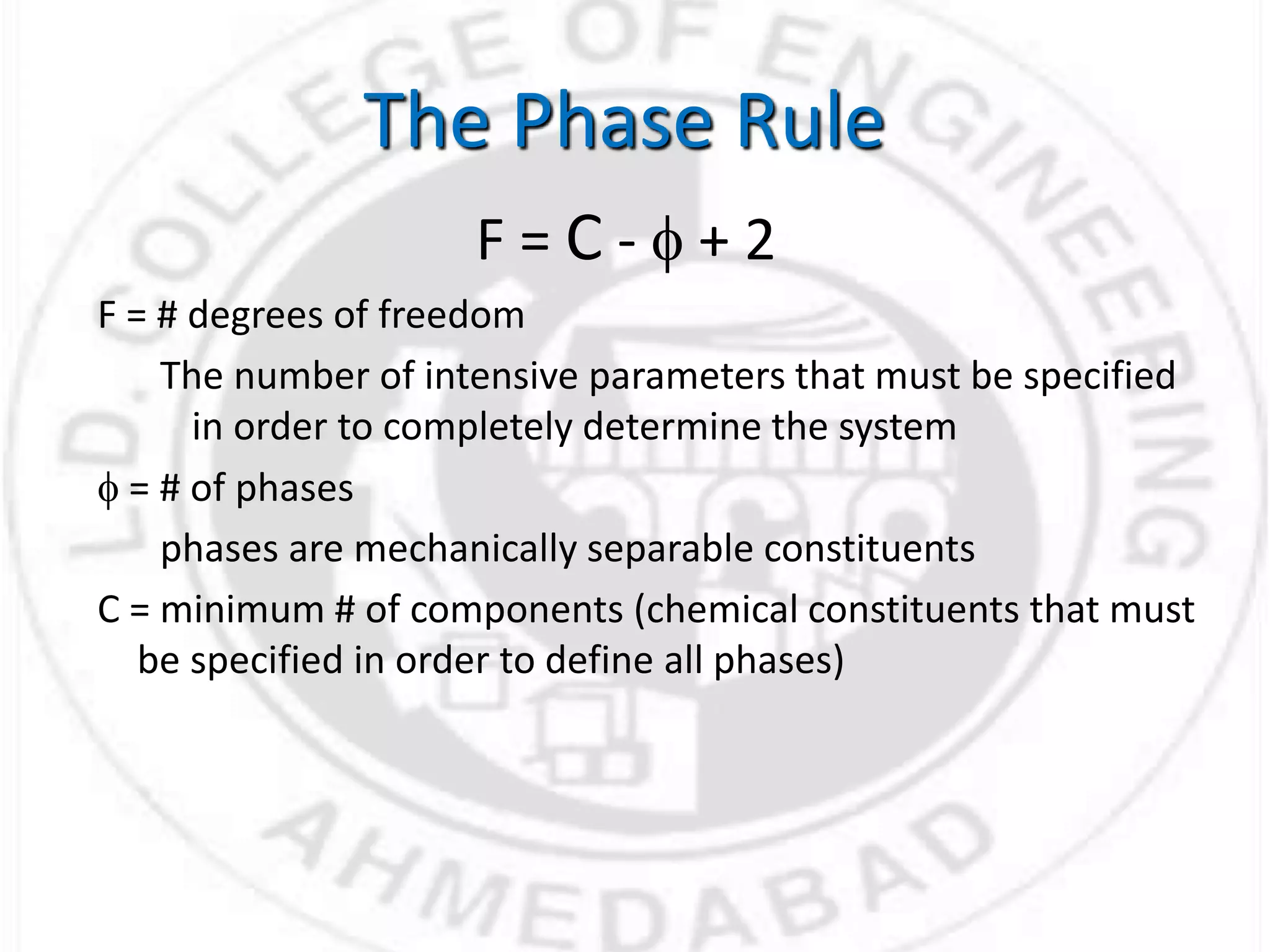
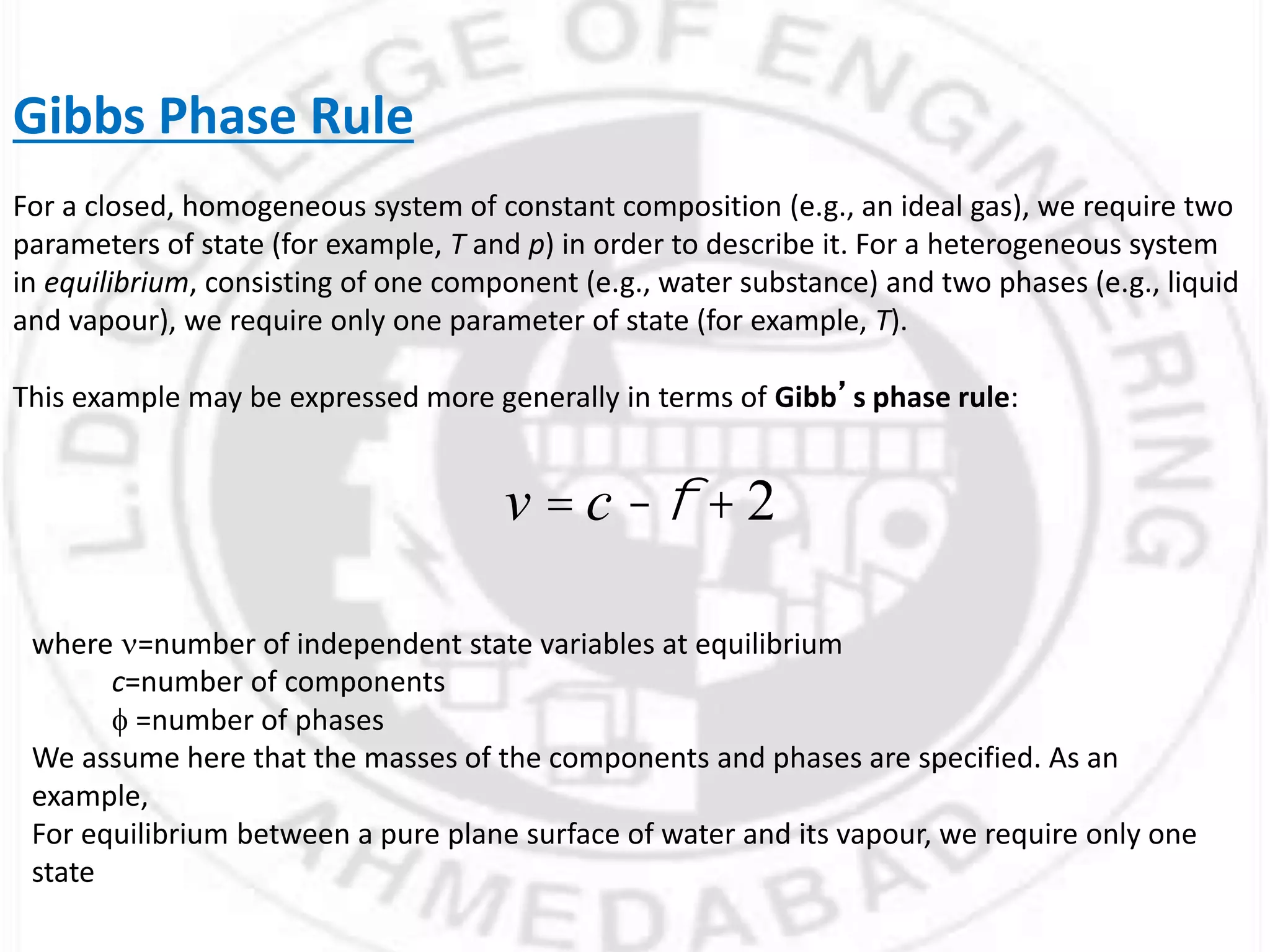
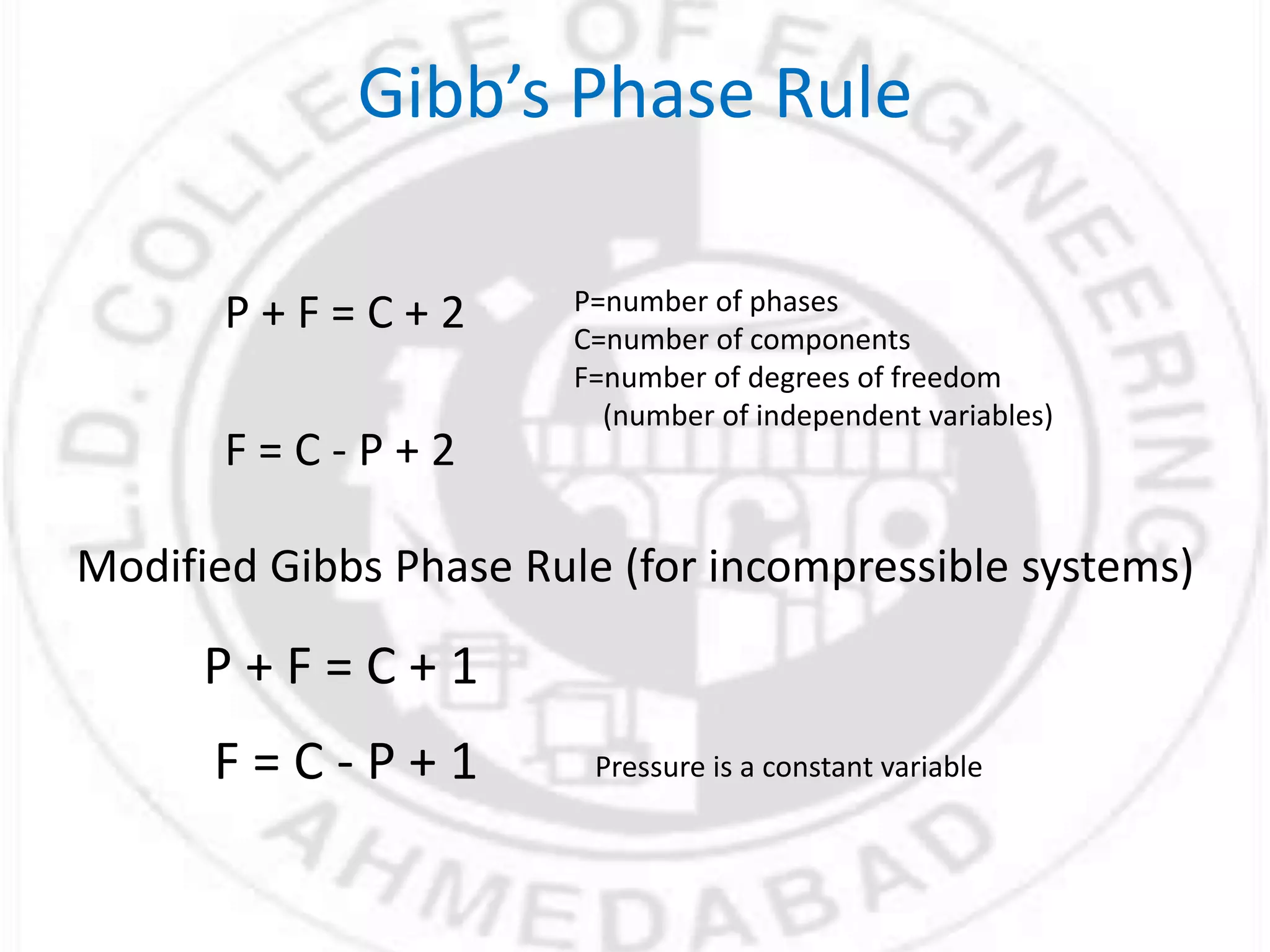
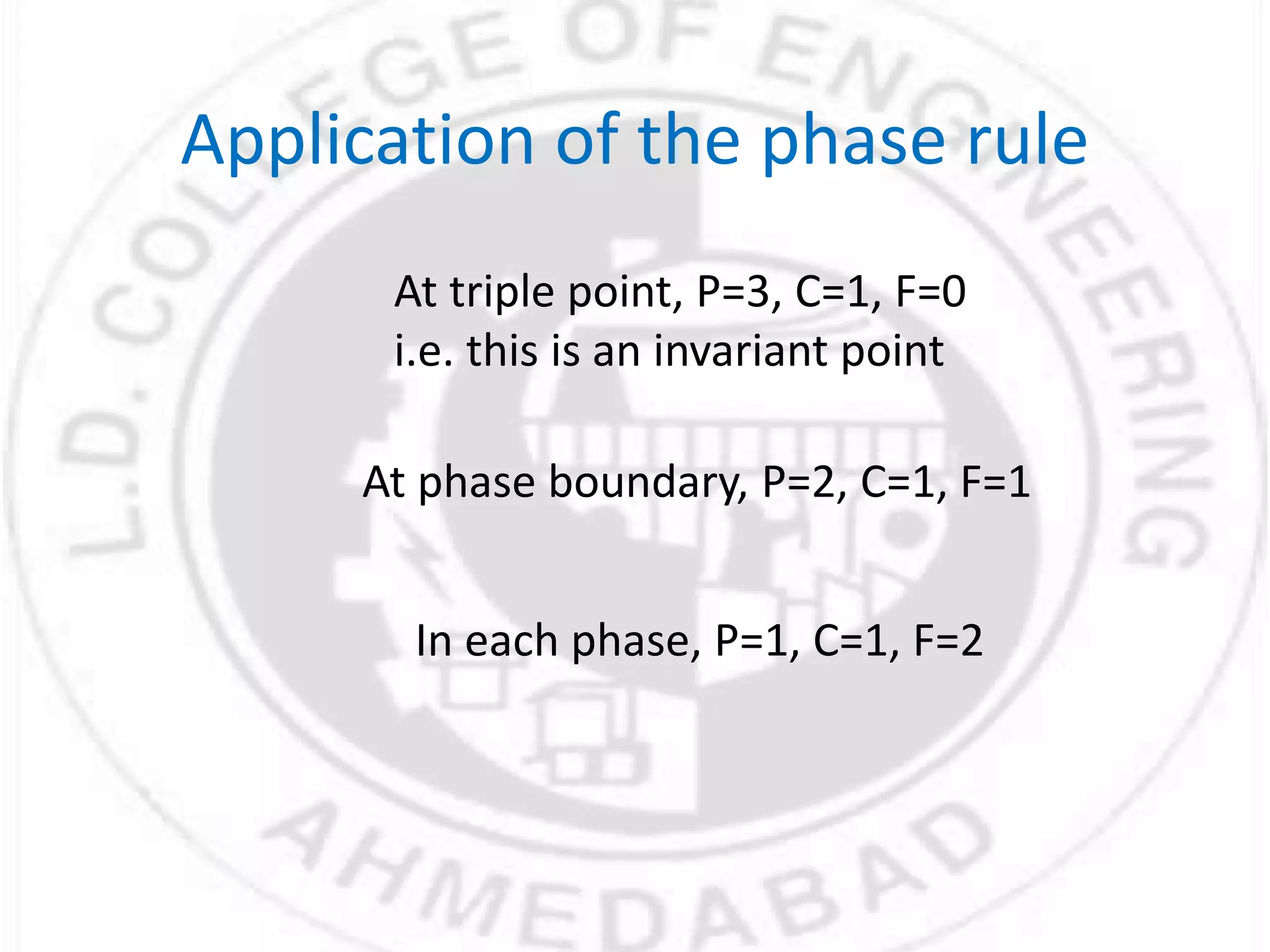
- Gibbs phase rule relates the number of phases (f), components (C), and degrees of freedom (F) using the equation F = C - f + 2. It describes the number of intensive parameters needed to determine a system at equilibrium.
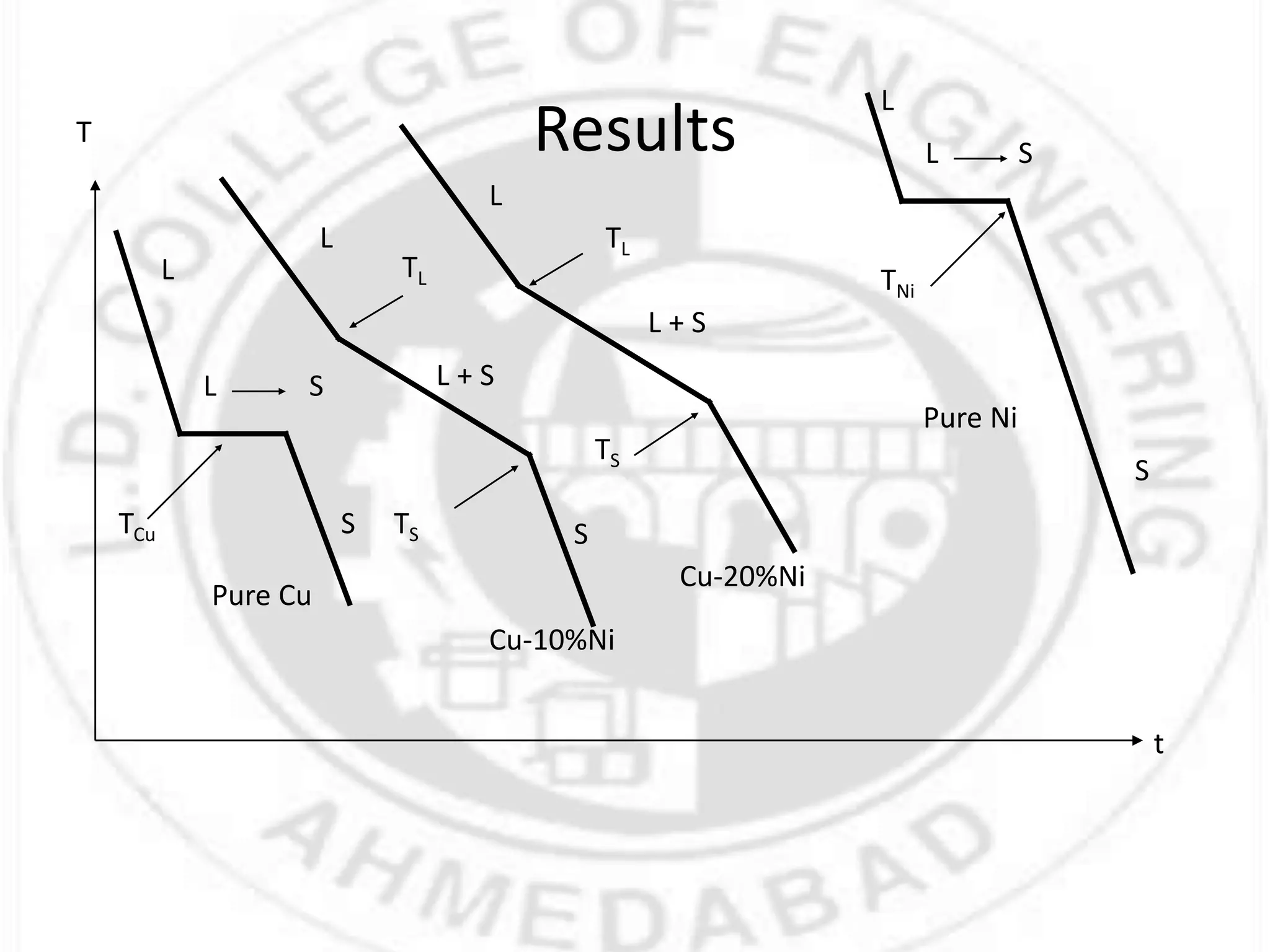
- For a single component system like water, equilibrium between the liquid and vapor phases requires only one state variable like temperature according to Gibbs phase rule. Phase diagrams can be constructed by conducting experiments on samples and recording phase