1. The periodic table organizes the chemical elements based on their properties. Dmitri Mendeleev published the first recognizable periodic table in 1869, arranging elements in order of atomic mass and leaving spaces for undiscovered elements.
2. In 1914, Henry Moseley redesigned the periodic table to be based on atomic number instead of atomic mass, solving inconsistencies and better organizing known and still-undiscovered elements.
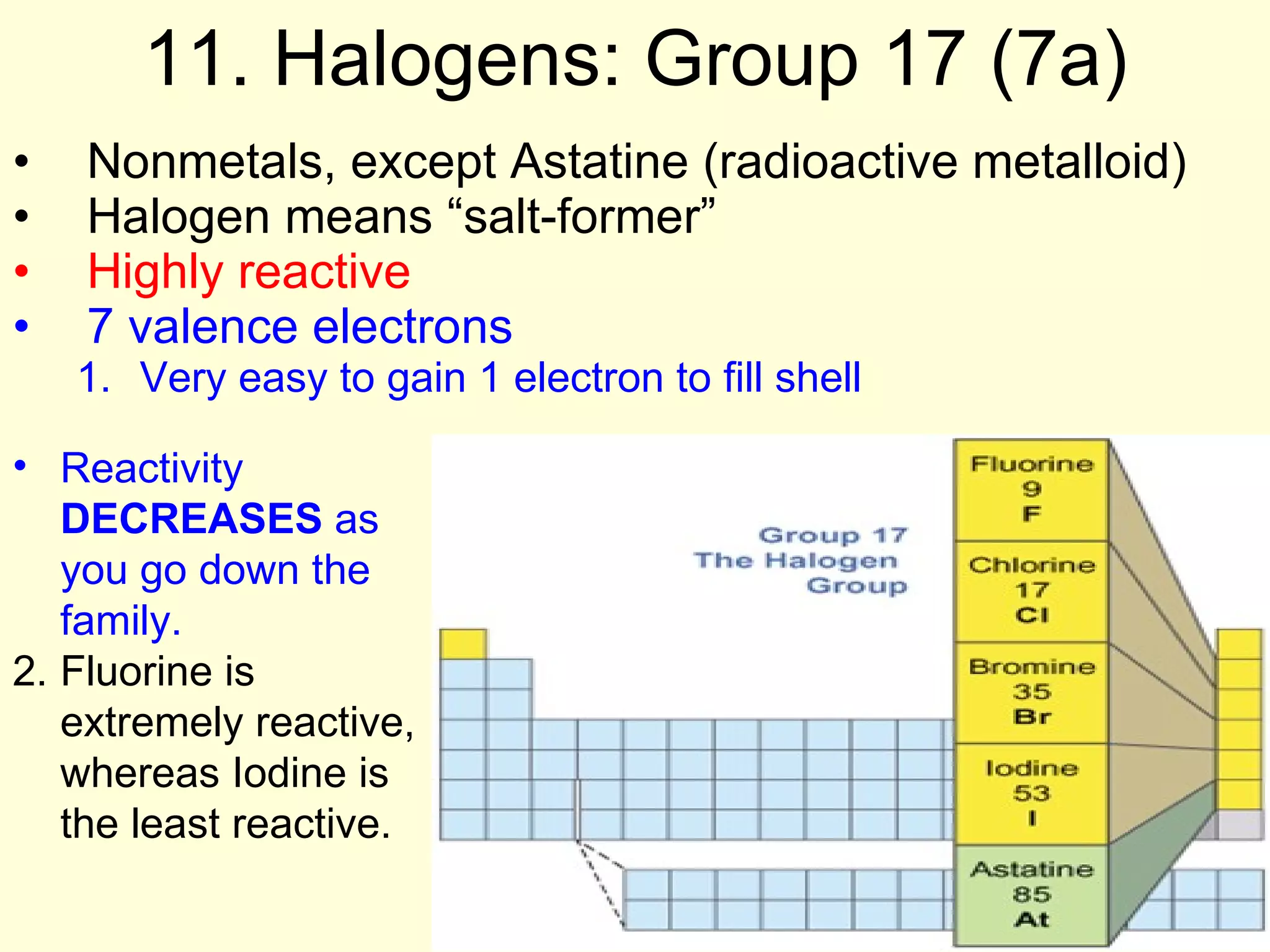
3. Elements are classified as metals, nonmetals, and metalloids, and are arranged into groups based on their valence electron configuration and chemical properties, with elements in the same group having similar reactivity.